Details of the Drug
General Information of Drug (ID: DM1794O)
| Drug Name |
Pyrethroids
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
resmethrin; 10453-86-8; Benzofuroline; For-syn; Penncapthrin; Resmethrine; Benzyfuroline; Isathrine; Enforcer; Chryson; Synthrin; Pyresthrin; Premgard; Crossfire; Chrysron; Resmethrin [ANSI]; Resbuthrin; Caswell No. 083E; Bioresmethrine; SB Pennick 1382; Penick 1382; Resmetrina [Portuguese]; ARI-B; Resmethrine [ISO-French]; S.B. Penick 1382; d-trans-Resmethrin; 5-Benzylfurfuryl chrysanthemate; NRDC 104; Bioresmethrin (d trans isomer); CCRIS 2501; HSDB 1516; SBP-1382; OMS-1206; EINECS 233-940-7; FMC 17370; ENT 27474; NIA 17370
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
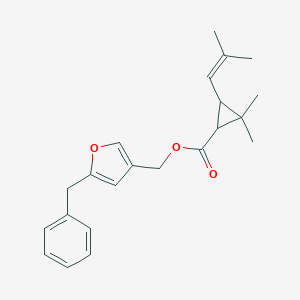 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 338.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
References
