Details of the Drug
General Information of Drug (ID: DM21C09)
| Drug Name |
Nitrendipine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Baylotensin; Bayotensin; Baypress; Bylotensin; Deiten; Gericin; Jutapress; Nidrel; Niprina; NitrePuren; Nitregamma; Nitrendepat; Nitrendidoc; Nitrendimerck; Nitrendipin; Nitrendipincorax; Nitrendipino; Nitrendipinum; Nitrensal; Nitrepin; Nitrepress; Tensogradal; Trendinol; Vastensium; Nitre AbZ; Nitre Puren; Nitren Lich;Nitren acis; Nitrend KSK; Nitrendi Biochemie; Nitrendipin AL; Nitrendipin Apogepha; Nitrendipin Atid; Nitrendipin Basics; Nitrendipin Heumann; Nitrendipin Lindo; Nitrendipin Stada; Nitrendipin beta; Nitrendipin corax; Nitrendipin von ct; Nitrendipino Bayvit; Nitren 1A Pharma; AL, Nitrendipin; AbZ, Nitre; Acis, Nitren; Atid, Nitrendipin; Basics, Nitrendipin; Baypress (TN); Bayvit, Nitrendipino; Beta, Nitrendipin; Biochemie, Nitrendi; Ct, nitrendipin von; Heumann, Nitrendipin; KSK, Nitrend; Lich, Nitren; Lindo, Nitrendipin; N-144; Nitre-Puren; Nitrendipin-corax; Pharma, Nitren 1A; Stada, Nitrendipin; Von ct, nitrendipin; Bay-e-5009; 1A Pharma, Nitren
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
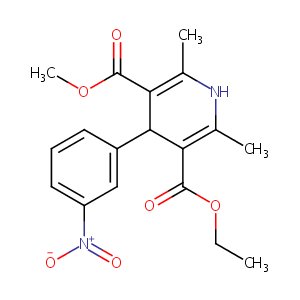 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 360.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hypertension | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA00-BA04 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
