Details of the Drug
General Information of Drug (ID: DM25FL8)
| Drug Name |
Methimazole
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
methimazole; 60-56-0; thiamazole; Tapazole; 2-Mercapto-1-methylimidazole; Mercazolyl; 1-Methylimidazole-2-thiol; Thymidazole; Mercazole; Methimazol; Thymidazol; Thiamazol; Metazolo; Thycapzol; Metothyrine; Metothyrin; Merkazolil; Mercaptazole; Strumazol; Favistan; Basolan; Thycapsol; Metotirin; Danantizol; Thacapzol; Merkastan; Frentirox; Metizol; Tiamazol; Mercasolyl; 1-Methyl-1H-imidazole-2-thiol; Methylmercaptoimidazole; 1-Methyl-2-mercaptoimidazole; Methiamazole; Usaf el-30; Methimazolum; Mercazolylum; Tapuzole; Metimazol; Thimazole; Basolan; MMZ; Mercazol; Methamazole; Methizol; Methymazol; Metisol; Strumazole; Thiamazole; Thiamazolum; Thimazol; Thyrozol; Tiamazolo; Tirodril; EliLilly Brand of Methimazole; Estedi Brand of Methimazole; Henning Berlin Brand of Methimazole; Hexal Brand of Methimazole; Jones Brand of Methimazole; Merck Brand of Methimazole; Nourypharma Brand of Methimazole; Philopharm Brand of Methimazole; Sanofi Synthelabo Brand of Methimazole; Temmler Brand of Methimazole; Thiamazol Henning; Thiamazol Hexal; Tiamazolo [DCIT]; M0868; Henning, Thiamazol; Hexal, Thiamazol; Methimazole (USP); Methimazole [USAN:BAN]; Tapazole (TN); Thiamazol [INN-French]; Thiamazolum [INN-Latin]; Tiamazol [INN-Spanish]; Mestinon, Regonol, Pyridostigmine Bromide;Thiamazole (JP15/INN); N-Methyl-2-mercaptoimidazole; 1 Methyl 2 mercaptoimidazole; 1,3-Dihydro-1-Methyl-2H-Imidazol-2-Thione; 1,3-Dihydro-1-methyl-2H-imidazole-2-thione; 1-METHYL-1,3-DIHYDRO-2H-IMIDAZOLE-2-THIONE; 1-Methyl-1,3-dihydroimidazole-2-thione; 1-Methyl-2-Imidazolethione; 1-Methyl-2-imidazolethiol; 1-Methyl-imidazole-2-thiol; 1-Methylimidazole-2(3H)-thione; 1-Metylo 2 merkaptoimidazolem; 1-Metylo 2 merkaptoimidazolem [Polish]; 2-Mercaptomethylimidazole; 3-methyl-1H-imidazole-2-thione
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antithyroid Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
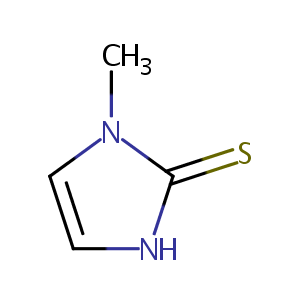 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 114.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hyperthyroidism | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A02 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Methimazole (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6649). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Diagnosis and treatment of thyrotoxicosis in childhood. A European questionnaire study. Eur J Endocrinol. 1994 Nov;131(5):467-73. | ||||
| 7 | Drug metabolism by flavin-containing monooxygenases of human and mouse. Expert Opin Drug Metab Toxicol. 2017 Feb;13(2):167-181. | ||||
| 8 | Low-expressional IGF1 mediated methimazole-induced liver developmental toxicity in fetal mice. Toxicology. 2018 Sep 1;408:70-79. doi: 10.1016/j.tox.2018.07.004. Epub 2018 Jul 7. | ||||
| 9 | Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17. | ||||
| 10 | Blood cell oxidative stress precedes hemolysis in whole blood-liver slice co-cultures of rat, dog, and human tissues. Toxicol Appl Pharmacol. 2010 May 1;244(3):354-65. doi: 10.1016/j.taap.2010.01.017. Epub 2010 Feb 6. | ||||
| 11 | Monitoring of deiodinase deficiency based on transcriptomic responses in SH-SY5Y cells. Arch Toxicol. 2013 Jun;87(6):1103-13. doi: 10.1007/s00204-013-1018-4. Epub 2013 Feb 10. | ||||
| 12 | Hepatocellular peroxisomes in human alcoholic and drug-induced hepatitis: a quantitative study. Hepatology. 1991 Nov;14(5):811-7. | ||||
| 13 | Role of CYP2A6 in Methimazole Bioactivation and Hepatotoxicity. Chem Res Toxicol. 2021 Dec 20;34(12):2534-2539. doi: 10.1021/acs.chemrestox.1c00300. Epub 2021 Nov 17. | ||||
| 14 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 15 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 16 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 17 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 18 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 19 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 20 | Canadian Pharmacists Association. | ||||
| 21 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 22 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 23 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
