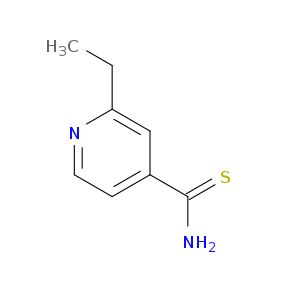
| DDI Drug Name |
DDI Drug ID |
Severity |
Mechanism |
Comorbidity |
REF |
| Remdesivir |
DMBFZ6L
|
Moderate |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Remdesivir. |
1D6YCoronavirus Disease 2019 [1D6YCoronavirus Disease 2019]
|
[5] |
| Repaglinide |
DM5SXUV
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Repaglinide. |
Acute diabete complication [5A2Y]
|
[6] |
| Glibenclamide |
DM8JXPZ
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Glibenclamide. |
Acute diabete complication [5A2Y]
|
[6] |
| Tolazamide |
DMIHRNA
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Tolazamide. |
Acute diabete complication [5A2Y]
|
[6] |
| Nateglinide |
DMLK2QH
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Nateglinide. |
Acute diabete complication [5A2Y]
|
[6] |
| Insulin-glulisine |
DMQI0FU
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Insulin-glulisine. |
Acute diabete complication [5A2Y]
|
[6] |
| Insulin-aspart |
DMX7V28
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Insulin-aspart. |
Acute diabete complication [5A2Y]
|
[7] |
| Glipizide |
DMZA5PQ
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Glipizide. |
Acute diabete complication [5A2Y]
|
[6] |
| Bedaquiline |
DM3906J
|
Moderate |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Bedaquiline. |
Antimicrobial drug resistance [MG50-MG52]
|
[8] |
| Pexidartinib |
DMS2J0Z
|
Major |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Pexidartinib. |
Bone/articular cartilage neoplasm [2F7B]
|
[9] |
| Ethanol |
DMDRQZU
|
Major |
Additive CNS depression effects by the combination of PMID28454500-Compound-96 and Ethanol. |
Cystitis [GC00]
|
[10] |
| Cannabidiol |
DM0659E
|
Moderate |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Cannabidiol. |
Epileptic encephalopathy [8A62]
|
[11] |
| Rifampin |
DMA8J1G
|
Major |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Rifampin. |
HIV-infected patients with tuberculosis [1B10-1B14]
|
[5] |
| Brentuximab vedotin |
DMWLC57
|
Moderate |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Brentuximab vedotin. |
Hodgkin lymphoma [2B30]
|
[12] |
| Efavirenz |
DMC0GSJ
|
Moderate |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Efavirenz. |
Human immunodeficiency virus disease [1C60-1C62]
|
[13] |
| Zalcitabine |
DMH7MUV
|
Moderate |
Increased risk of peripheral neuropathy by the combination of PMID28454500-Compound-96 and Zalcitabine. |
Human immunodeficiency virus disease [1C60-1C62]
|
[14] |
| Mipomersen |
DMGSRN1
|
Major |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Mipomersen. |
Hyper-lipoproteinaemia [5C80]
|
[15] |
| Teriflunomide |
DMQ2FKJ
|
Major |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Teriflunomide. |
Hyper-lipoproteinaemia [5C80]
|
[16] |
| BMS-201038 |
DMQTAGO
|
Major |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and BMS-201038. |
Hyper-lipoproteinaemia [5C80]
|
[17] |
| Calaspargase pegol |
DMQZBXI
|
Moderate |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Calaspargase pegol. |
Malignant haematopoietic neoplasm [2B33]
|
[18] |
| Idelalisib |
DM602WT
|
Moderate |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Idelalisib. |
Mature B-cell leukaemia [2A82]
|
[19] |
| Clofarabine |
DMCVJ86
|
Moderate |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Clofarabine. |
Mature B-cell lymphoma [2A85]
|
[20] |
| Thalidomide |
DM70BU5
|
Moderate |
Increased risk of peripheral neuropathy by the combination of PMID28454500-Compound-96 and Thalidomide. |
Multiple myeloma [2A83]
|
[14] |
| Leflunomide |
DMR8ONJ
|
Major |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Leflunomide. |
Rheumatoid arthritis [FA20]
|
[16] |
| Trabectedin |
DMG3Y89
|
Moderate |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Trabectedin. |
Solid tumour/cancer [2A00-2F9Z]
|
[11] |
| Naltrexone |
DMUL45H
|
Moderate |
Increased risk of hepatotoxicity by the combination of PMID28454500-Compound-96 and Naltrexone. |
Substance abuse [6C40]
|
[21] |
| Tolbutamide |
DM02AWV
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Tolbutamide. |
Type 2 diabetes mellitus [5A11]
|
[6] |
| Chlorpropamide |
DMPHZQE
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Chlorpropamide. |
Type 2 diabetes mellitus [5A11]
|
[6] |
| Insulin-detemir |
DMOA4VW
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Insulin-detemir. |
Type-1/2 diabete [5A10-5A11]
|
[6] |
| Insulin degludec |
DMPL395
|
Moderate |
Increased risk of hypoglycemia by the combination of PMID28454500-Compound-96 and Insulin degludec. |
Type-1/2 diabete [5A10-5A11]
|
[6] |
| ----------- |
|
|
|
|
|