Details of the Drug
General Information of Drug (ID: DMH7MUV)
| Drug Name |
Zalcitabine
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
zalcitabine; Dideoxycytidine; 7481-89-2; 2',3'-DIDEOXYCYTIDINE; ddCyd; HIVID; ddC; Cytidine, 2',3'-dideoxy-; Zalcitibine; 4-Amino-1-((2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one; 2,3-dideoxycytidine; NSC 606170; UNII-6L3XT8CB3I; CCRIS 692; HSDB 7156; C9H13N3O3; Ro-24-2027/000; Ro 24-2027/000; CHEMBL853; BRN 0654956; 6L3XT8CB3I; CHEBI:10101; 1-(2,3-Dideoxy-beta-D-ribofuranosyl)cytosine; WREGKURFCTUGRC-POYBYMJQSA-N; 4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one; MFCD00012188; DdC; DdCyd; D 5782; DDC (DDC); DdC & Interferon alpha; DdC & sCD4; DdC (Antiviral); Hivid (TN); Hivid(TM); Interferon AD + ddC; KS-1130; SRI-7707; Beta-D-DDC; DS-4152 & ddC; DdC & GM-CSF; DdC & IFN-alpha; DdC & NP (from PHCA or HSA); PC-SOD & ddC; Zalcitabine [USAN:INN:BAN]; Hivid, Dideoxycytidine, NSC 606170, Zalcitabine; Zalcitabine (JAN/USP/INN); Beta-D-2',3'-Dideoxycytidine; Cytidine, 2',3'-dideoxy & Interferon alpha; Sulfated polysaccharide-peptidoglycan DS-4152 & 2',3'-Dideoxycytidine; Cytidine, 2',3'-dideoxy-& Colony-stimulating factor; Beta-D-2',3'-Dideoxycytidine & Granulocyte-macrophage colony-stimulating factor; Lecithinized superoxide dismutase & beta-D-2',3'-Dideoxycytidine; 2',3'-Dideoxycytidine & Interferon-alpha; 2',3'-Dideoxycytidine & Nanoparticles (from human serum albumin or polyhexylcyanoacrylate); 2',3'-Dideoxycytidine & sCD4(soluble recombinant protein); 3'-Azido-3'-deoxythymidine/2',3'-Dideoxycytidine; 4-amino-1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one; DdC
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Human Immunodeficiency Virus
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
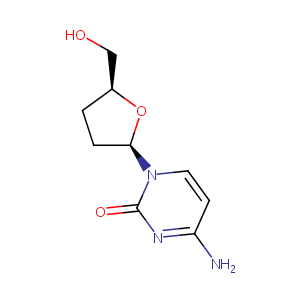 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 211.22 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Zalcitabine
Coadministration of a Drug Treating the Disease Different from Zalcitabine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4828). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 6 | A peptide inhibitor of HIV-1 reverse transcriptase using alpha,beta-dehydro residues: a structure-based computer model. J Biomol Struct Dyn. 1998 Oct;16(2):347-54. | ||||
| 7 | MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2',3'-dideoxycytidine and 9'-(2'-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003 Aug 8;278(32):29509-14. | ||||
| 8 | Interaction of zalcitabine with human organic anion transporter 1. Pharmazie. 2006 May;61(5):491-2. | ||||
| 9 | Protease inhibitors in patients with HIV disease. Clinically important pharmacokinetic considerations. Clin Pharmacokinet. 1997 Mar;32(3):194-209. | ||||
| 10 | Binding of anti-HIV drugs to human serum albumin. IUBMB Life. 2004 Oct;56(10):609-14. doi: 10.1080/15216540400016286. | ||||
| 11 | Assessment of mitochondrial toxicity by analysis of mitochondrial protein expression in mononuclear cells. Cytometry B Clin Cytom. 2009 May;76(3):181-90. doi: 10.1002/cyto.b.20458. | ||||
| 12 | Small-scale immunopurification of cytochrome c oxidase for a high-throughput multiplexing analysis of enzyme activity and amount. Biotechnol Appl Biochem. 2007 Dec;48(Pt 4):167-78. doi: 10.1042/BA20060223. | ||||
| 13 | Molecular basis of 2',3'-dideoxycytidine-induced drug resistance in human cells. Mol Cell Biochem. 2002 Feb;231(1-2):173-7. doi: 10.1023/a:1014441209108. | ||||
| 14 | A dominant-negative form of the major human abasic endonuclease enhances cellular sensitivity to laboratory and clinical DNA-damaging agents. Mol Cancer Res. 2007 Jan;5(1):61-70. doi: 10.1158/1541-7786.MCR-06-0329. | ||||
| 15 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 16 | Argov Z, Mastaglia FL "Drug-induced peripheral neuropathies." Br Med J 1 (1979): 663-6. [PMID: 219931] | ||||
| 17 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 18 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 19 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 20 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 21 | Canadian Pharmacists Association. | ||||
| 22 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 23 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 24 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 25 | MHRA. Medicines and Healthcare Products Regulatory Agency "Orlistat: theoretical interaction with antiretroviral HIV medicines.". | ||||
