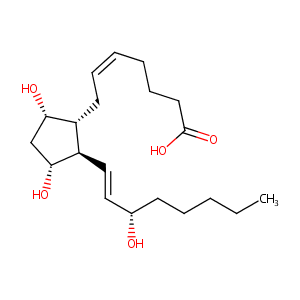
| 1 |
Stereocontrolled organocatalytic synthesis of prostaglandin PGF2alpha in seven steps. Nature. 2012 Sep 13;489(7415):278-81.
|
| 2 |
The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000 Jan 17;1483(2):285-93.
|
| 3 |
Expression and molecular pharmacology of the mouse CRTH2 receptor. J Pharmacol Exp Ther. 2003 Aug;306(2):463-70.
|
| 4 |
Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol. 1997 Dec 11;340(2-3):227-41.
|
| 5 |
Importance of the extracellular domain for prostaglandin EP(2) receptor function. Mol Pharmacol. 1999 Sep;56(3):545-51.
|
| 6 |
Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997 Sep;122(2):217-24.
|
| 7 |
Identification by site-directed mutagenesis of amino acids contributing to ligand-binding specificity or signal transduction properties of the human FP prostanoid receptor. Biochem J. 2003 Apr 15;371(Pt 2):443-9.
|
| 8 |
Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA(hPGT). J Clin Invest. 1996 Sep 1;98(5):1142-9.
|
| 9 |
Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol. 2003 Dec;285(6):F1188-97.
|
| 10 |
Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. J Pharmacol Exp Ther. 2002 Jun;301(3):797-802.
|
| 11 |
Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J Pharmacol Exp Ther. 2002 Apr;301(1):293-8.
|
| 12 |
Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007.
|
| 13 |
Metabolism of prostaglandins by the nonpregnant human uterus. J Clin Endocrinol Metab. 1983 Apr;56(4):678-85.
|
| 14 |
Prostaglandins and leukotriene B4 are potent inhibitors of 11beta-hydroxysteroid dehydrogenase type 2 activity in human choriocarcinoma JEG-3 cells. Biol Reprod. 1999 Jul;61(1):40-5.
|
| 15 |
Identification of the major prostaglandin glycerol ester hydrolase in human cancer cells. J Biol Chem. 2014 Dec 5;289(49):33741-53. doi: 10.1074/jbc.M114.582353. Epub 2014 Oct 9.
|
| 16 |
Relationship of human liver dihydrodiol dehydrogenases to hepatic bile-acid-binding protein and an oxidoreductase of human colon cells. Biochem J. 1996 Jan 15;313 ( Pt 2)(Pt 2):373-6.
|
| 17 |
Expression of cyclooxygenase genes and production of prostaglandins during ovulation in the ovarian follicles of Xenopus laevis. Gen Comp Endocrinol. 2008 Jun;157(2):165-73. doi: 10.1016/j.ygcen.2008.04.012. Epub 2008 May 1.
|
| 18 |
Inactivation of lipid glyceryl ester metabolism in human THP1 monocytes/macrophages by activated organophosphorus insecticides: role of carboxylesterases 1 and 2. Chem Res Toxicol. 2010 Dec 20;23(12):1890-904. doi: 10.1021/tx1002194.
|
| 19 |
Effects of PCB126 and 17beta-oestradiol on endothelium-derived vasoactive factors in human endothelial cells. Toxicology. 2011 Jul 11;285(1-2):46-56.
|
| 20 |
Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003 Jan;188(1):252-63. doi: 10.1067/mob.2003.70.
|
| 21 |
Suppressive effects of antimycotics on thymic stromal lymphopoietin production in human keratinocytes. J Dermatol Sci. 2013 Sep;71(3):174-83. doi: 10.1016/j.jdermsci.2013.04.023. Epub 2013 May 2.
|
|
|
|
|
|
|