Details of the Drug
General Information of Drug (ID: DM5QSKN)
| Drug Name |
INDOPROFEN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dexindoprofen; UNII-004T8726AU; 53086-13-8; 004T8726AU; (+)-indoprofen; Dexindoprofenum; Dexindoprofene; Dexindoprofeno; Dexindoprofen [INN]; Dexindoprofenum [INN-Latin]; Dexindoprofene [INN-French]; Dexindoprofeno [INN-Spanish]; Indoprofen (+)-; EINECS 258-351-2; AC1Q5QZO; AC1L2A9M; ZINC391; SCHEMBL286896; (+)-(S)-4-(1-Oxo-2-isoindolinyl)hydratropasaeure; CHEMBL2106252; AJ-07888; (+)-(S)-p-(1-Oxo-2-isoindolinyl)hydratropic acid; UNII-CPE46ZU14N component RJMIEHBSYVWVIN-NSHDSACASA-N
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
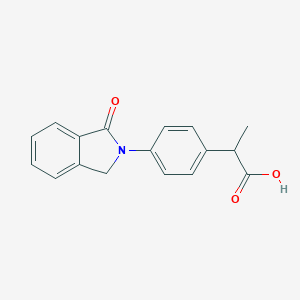 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 281.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Gout | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | FA25 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
