Details of the Drug
General Information of Drug (ID: DM6HM5S)
| Drug Name |
N1-(2-aminoethyl)ethane-1,2-diamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
DIETHYLENETRIAMINE; 111-40-0; Bis(2-aminoethyl)amine; 2,2'-Diaminodiethylamine; Diethylene triamine; Barsamide 115; Epicure T; Ancamine DETA; 1,4,7-Triazaheptane; 2,2'-Iminodiethylamine; N,N-Bis(2-aminoethyl)amine; Aminoethylethandiamine; 3-Azapentane-1,5-diamine; 1,2-Ethanediamine, N-(2-aminoethyl)-; Imino-bis-ethylamine; ChS-P 1; N-(2-Aminoethyl)-1,2-ethanediamine; N-(2-aminoethyl)ethane-1,2-diamine; 1,5-Diamino-3-azapentane; 2,2'-Iminobis(ethanamine); Bis(beta-aminoethyl)amine; 2-(2-Aminoethylamino)ethylamine; Epon 3223
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
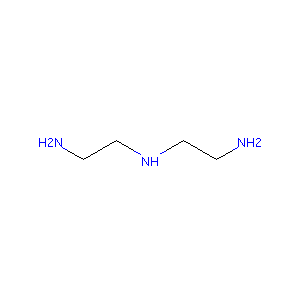 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 103.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
References
