Details of the Drug
General Information of Drug (ID: DM7583A)
| Drug Name |
Beta-caryophyllene
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
BETA-CARYOPHYLLENE; Caryophyllene; (-)-trans-Caryophyllene; 87-44-5; L-Caryophyllene; (-)-beta-caryophyllene; trans-Caryophyllene; (E)-Caryophyllene; (E)-beta-caryophyllene; b-caryophyllene; trans-beta-caryophyllene; Beta-Caryophylene; (-)-Caryophyllene; beta-Caryophyllen; beta-Caryophyllene; CHEBI:10357; (E)-beta-caryophylene; beta-(E)-Caryophyllene; beta-Caryophyllen; (1R,4E,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene; MFCD00075925; UNII-BHW853AU9H; g-Caryophyllene; (1R,9S,E)-4,11,11-trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene; (1S,9R)-6,10,10-trimethyl-2-methylenebicyclo[7.2.0]undec-5-ene; NSC 11906; E-.beta.-caryophyllene; BHW853AU9H; beta-Caryophyllene, (-); Tincturoid; NSC11906; Caryophyllene B; beta-cariofillene; (1R,4E,9S)-4,11,11-trimethyl-8-methylene-bicyclo[7.2.0]undec-4-ene; beta-caryophillene; Bicyclo(7.2.0)undec-4-ene, 8-methylene-4,11,11-trimethyl-, (E)-(1R,9S)-(-)-; Bicyclo[7.2.0]undec-4-ene, 8-methylene-4,11,11-trimethyl-, (E)-(1R,9S)-(-)-; Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-, [1R-(1R*,4E,9S*)]-; E-beta-caryophyllene; Caryophyllene, (E); beta-(e)-caryophyllene; beta-trans-caryophyllene; (-)- -caryophyllene; (-)-(E)-Caryophyllene; DSSTox_CID_4739; beta-trans-Caryophyllene; trans-.beta.-Caryophyllene; (-)-I(2)-caryophyllene; DSSTox_RID_77517; DSSTox_GSID_24739; 8-Methylene-4,11,11-(trimethyl)bicyclo[7.2.0]undec-4-ene; CHEMBL445740; DTXSID8024739; HY-N1415; ZINC8234282; Tox21_301497; BDBM50529607; s6058; (1R,4E,9S)-4,11,11-trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene; AKOS024283988; trans-(1R,9S)-8-Methylene-4,11,11-trimethylbicyclo[7.2.0]undec-4-ene; LMPR0103120001; beta-Caryophyllene, >=80%, FCC, FG; CAS-87-44-5; NCGC00142620-01; NCGC00255159-01; ST072181; (-)-trans-Caryophyllene, analytical standard; CS-0016839; V0915; C09629; Q421614; W-109317; UNII-K4H93P747O component NPNUFJAVOOONJE-GFUGXAQUSA-N; (-)-trans-Caryophyllene, >=98.5% (sum of enantiomers, GC); trans-(1R,9S)-4,11,11-trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene; 8-Methylene-4,11,11-(trimethyl)bicyclo(7.2.0)undec-4-ene, (1R,4E,9S)-; Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-, (E)-(1R,9S)-(-)-; Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-, [1R- (1R*,4E,9S*)]-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
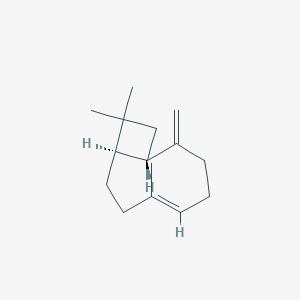 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 204.35 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 0 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Pain | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | MG30-MG3Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
