Details of the Drug
General Information of Drug (ID: DM7R3B6)
| Drug Name |
Nobiletin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
478-01-3; Hexamethoxyflavone; 3',4',5,6,7,8-Hexamethoxyflavone; 5,6,7,8,3',4'-Hexamethoxyflavone; UNII-D65ILJ7WLY; 2-(3,4-Dimethoxyphenyl)-5,6,7,8-tetramethoxy-4H-1-benzopyran-4-one; 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxychromen-4-one; NSC-76751; D65ILJ7WLY; 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-4H-chromen-4-one; CHEMBL76447; Nobiletin (Hexamethoxyflavone); CHEBI:7602; NSC76751; MFCD03273560; Flavone, 5,6,7,8,3',4'-hexamethoxy; 4H-1-Benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-; SMR000156231; CCRIS 9012; NSC 76751; CPD000156231; Nobiletin, >=97%; Spectrum2_001697; Spectrum3_000921; Spectrum4_001020; KBioGR_001519; MLS000574877; MLS000759462; MLS000877030; MLS001424129; Nobiletin, analytical standard; SCHEMBL244029; SPECTRUM1505268; SPBio_001654; MEGxp0_000930; ACon1_000921; KBio3_001922; DTXSID30197275; 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-chromen-4-one; HMS2051D09; HMS2234A09; HMS3373C14; HMS3393D09; HMS3651G20; HY-N0155; ZINC1531669; 3'4'5,6,7,8-Hexamethoxyflavone; 3,4,5,6,7,8-Hexamethoxyflavone; ANW-42631; BDBM50338976; CCG-38781; CN0043; LMPK12111468; NSC618903; STL565829; AKOS015965334; NOBILETIN, 20% (Technical Grade); AC-1023; CS-5518; MCULE-1015144950; NC00186; NSC-618903; SDCCGMLS-0066776.P001; NCGC00095703-01; NCGC00095703-02; NCGC00169228-01; 5,6,7,8,3'',4''-hexamethoxyflavone; AK168175; AS-17452; NCI60_041691; DB-050181; FT-0686667; N0871; N1311; S2333; SW197566-2; V0181; C10112; SR-01000712262; Q-100511; Q2402963; SR-01000712262-5; BRD-K06753942-001-02-0; 2-(3,4-Dimethoxy-phenyl)-5,6,7,8-tetramethoxy-chromen-4-one; 4H-1-Benzopyran-4-one,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-; 2-(3,4-Dimethoxyphenyl)-5,6,7,8-tetramethoxy-4H-1-benzopyran-4-one, 9CI; 3 inverted exclamation mark ,4 inverted exclamation mark ,5,6,7,8-HEXAMETHOXYFLAVONE; 4H-1-Benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy- (9CI)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
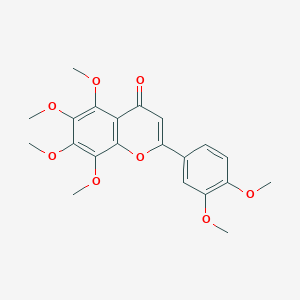 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 402.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
