Details of the Drug
General Information of Drug (ID: DM7V9CT)
| Drug Name |
GM6001
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ilomastat; Galardin; 142880-36-2; GM 6001; Illomastat; CS 610; GM-6001; UNII-I0403ML141; CHEMBL19611; Ilomastat (GM6001, Galardin); I0403ML141; NCGC00163450-02; 3-(N-HYDROXYCARBOXAMIDO)-2-ISOBUTYLPROPANOYL-TRP-METHYLAMIDE; (R)-N4-Hydroxy-N1-[(S)-2-(1H-indol-3-yl)-1-methylcarbamoyl-ethyl]-2-isobutyl-succinamide; N-[(2R)-2-(Hydroxamidocarbonylmethyl)-4-methylpentanoyl]-L-tryptophan Methylamide; (R)-N(sup 1)-Hydroxy-N-((S)-2-indol-3-yl-1-(methylcarbamoyl)ethyl)-2-isobutylsuccinamide; (S-(R*,S*))-N(sup 4)-Hydroxy-N(sup; ILOMASTAT
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
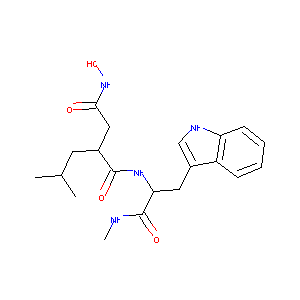 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 388.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
