Details of the Drug
General Information of Drug (ID: DM8DZC1)
| Drug Name |
Dyphylline
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aristophyllin; Asthmolysin; Astmamasit; Astrophyllin; Circain; Circair; Coronal; Coronarin; Corphyllin; Dihydroxypropyltheophylline; Diprofilina; Diprofillin; Diprofillina; Diprofilline; Diprophyllin; Diprophylline; Diprophyllinum; Dipropylline; Droxine; Dyflex; Glyfyllin; Glyphyllin; Glyphylline; Glyphyllinum; Hidroxiteofillina; Hiphyllin; Hyphylline; Iphyllin; Isophyllen; Liactemin; Lufyllin; Neophyl; Neophyllin; Neophylline; Neostenovasan; Neothylline; Neotilina; Neufil; Neutrafil; Neutrafillina; Neutraphyllin; Neutraphylline; Neutroxantina; Propyphyllin; Protheophylline; Purifilin; Silbephyllin; Silbephylline; Solufilin; Solufyllin; Soluphyllin; Synthophylline; Tefilan; Teofen; Tesfen; Theal; Thefylan; Dihydroxypropyl theophylline; Dihydroxypropyl theopylin; Dilor G; Diprofillina [DCIT]; Dyphylline [USAN]; Neophyllin M; Theal ampules; AFI-Phyllin; COR-theophylline; Dihydroxypropyl theopylin (german); Dilor-400; Diphyllin (VAN); Diprofilina [INN-Spanish]; Diprophyllinum [INN-Latin]; Dyphylline (USP); Lufyllin (TN); Neo-Vasophylline; Diprophylline (JAN/INN); Neothylline, Lufyllin, diprophylline,Dyphylline; (+-)-7-(2,3-Dihydroxypropyl)theophylline; (+-)-diprophylline; (+-)-dyphylline; (1,2-Dihydroxy-3-propyl)thiophyllin; 1,3-Dimethyl-7-(2,3-dihydroxypropyl)xanthine; 1H-Purine-2,6-dione, 7-(2,3-dihydroxypropyl)-3,7-dihydro-1,3-dimethyl; 7-(2,3-Dihydroxypropyl)-1,3-dimethyl-3,7-dihydro-1H-purine-2,6-dione; 7-(2,3-Dihydroxypropyl)-1,3-dimethylxanthine; 7-(2,3-Dihydroxypropyl)-3,7-dihydro-1,3-dimethyl-1H-purine-2,6-dione; 7-(2,3-Dihydroxypropyl)theophylline; 7-(2,3-Dioxypropyl)theophylline; 7-(2,3-dihydroxypropyl)-1,3-dimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-dione; 7-(2,3-dihydroxypropyl)-1,3-dimethylpurine-2,6-dione; 7-(beta,gamma-Dihydroxypropyl)theophylline; 7-(beta.,.gamma.-Dihydroxypropyl)theophylline; 7-DIHYDROXYPROPYLTHEOPHYLLINE; 7-[2,3-Dihydroxypropyl]-theophylline
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Bronchodilator Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
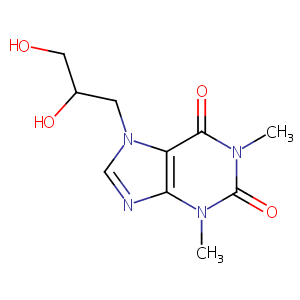 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 254.24 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.8 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Acute bronchial asthma | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA23 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Dyphylline (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||
References
