Details of the Drug
General Information of Drug (ID: DM9AFZ3)
| Drug Name |
CL-5343
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5-Amino-1,3,4-thiadiazole-2-sulfonamide; 14949-00-9; Tio-urasin; CL 5343; UNII-F687N81LIZ; NSC 22979; 2-Amino-1,3,4-thiadiazole-5-sulfonamide; CHEMBL265674; F687N81LIZ; 1,3,4-Thiadiazole-2-sulfonamide, 5-amino-; 5-Amino-TDSNH2; PubChem15758; Acetazolamide Impurity D; AC1Q6UUX; 1,3,4-Thiadiazole-5-sulfonamide, 2-amino-; SCHEMBL282413; AC1L382D; CTK4C6227; BDBM10868; DTXSID10164324; MolPort-022-374-081; VGMVBPQOACUDRU-UHFFFAOYSA-N; NSC22979; ZINC16969869; NSC-22979; 1,3,4-thiadiazole-2-sulfonamide 15; aromatic/heteroaromatic; CL-5343; Carbonic anhydrase inhibitors, Universita degli Studi di Firenze; Sulfonamide CA inhibitors, Universita degli Studi di Firenze; Sulfonamide CA inhibitors, University of Florence; Carbonic anhydrase inhibitors (cancer/epilepsy/glaucoma/obesity); Carbonic anhydrase inhibitors (cancer/epilepsy/glaucoma/obesity), University of Florence/ULS; 5-amino-1,3,4-thiadiazole-2-sulfonamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
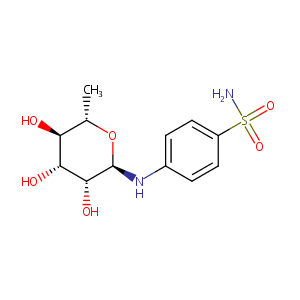 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 180.21 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
