| Drug Name |
Hydroxybenzo(a)pyrene
|
| Synonyms |
3-Hydroxybenzo(a)pyrene; Benzo(a)pyrene, 3-hydroxy-; Benzo[def]chrysen-3-ol; Benzo[pqr]tetraphen-3-ol; SCHEMBL145489; benzo[a]pyren-3-ol; 13345-21-6; 3-Hydroxy Benzopyrene; 3-Hydroxy benzo[a]pyrene; 3-Hydroxy-3,4-benzo[a]pyrene; 3-Hydroxybenzo[a]pyrene; 3-hydroxybenz[a]pyrene; 4-06-00-05133 (Beilstein Handbook Reference); 672ICH1Q4L; 8-Hydroxy-3,4-benzpyrene; BENZO(a)PYREN-3-OL; AC1L19Z3; BIDD:ER0040; BP-3-Hydroxy; BRN 2333868; CCRIS 1071; CHEBI:34337; CHEMBL8020; CTK0H7534; DTXSID7038319; UNII-672ICH1Q4L
|
| Structure |
|
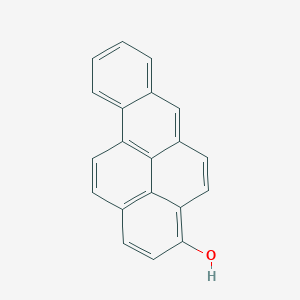 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight (mw) |
268.3 |
|
| Logarithm of the Partition Coefficient (xlogp) |
5.9 |
| Rotatable Bond Count (rotbonds) |
0 |
| Hydrogen Bond Donor Count (hbonddonor) |
1 |
| Hydrogen Bond Acceptor Count (hbondacc) |
1 |
| Chemical Identifiers |
- Formula
- C20H12O
- IUPAC Name
benzo[a]pyren-3-ol - Canonical SMILES
-
C1=CC=C2C3=C4C(=CC2=C1)C=CC5=C(C=CC(=C54)C=C3)O
- InChI
-
SPUUWWRWIAEPDB-UHFFFAOYSA-N
- InChIKey
-
1S/C20H12O/c21-18-10-7-12-5-8-16-15-4-2-1-3-13(15)11-14-6-9-17(18)19(12)20(14)16/h1-11,21H
|
| Cross-matching ID |
- PubChem CID
- 25890
- ChEBI ID
-
- CAS Number
-
- INTEDE ID
- DR1965
|
|
|
|
|
|
|
|



