Details of the Drug
General Information of Drug (ID: DM9Y6X7)
| Drug Name |
Clodronate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bonefos; Clodronsaeure; Dichloromethanediphosphonate; Dichloromethylenebisphosphonate; Methanedichlorodiphosphonate; Acide clodronique; Acido clodronico; Acidum clodronicum; Clodronic Acid; Dichloromethanediphosphonic acid; Dichloromethylene Biphosphonate; Dichloromethylene Diphosphonate; Dichloromethylidene diphosphonate; Liposomes containing clodronic acid; Methanedichlorodiphosphonic acid; Acid, Clodronic; Acid, Dichloromethanediphosphonic; Acide clodronique [INN-French]; Acido clodronico [INN-Spanish]; Acidum clodronicum [INN-Latin]; Biphosphonate, Dichloromethylene; Bonefos (TN); Clodron (TN); Diphosphonate, Dichloromethane; Diphosphonate, Dichloromethylene; Disodium, Clodronate; Loron (TN); Sodium, Clodronate; [dichloro(phosphono)methyl]phosphonic acid; Clodronic acid (USAN/INN); Clodronic acid [USAN:BAN:INN]; Dichlormethylen-bis(phosphonsaeure); Dichloromethylene-1,1-bisphosphonic acid; Dichloromethylene-1,1-diphosphonic acid; Phosphonic acid, (dichloromethylene)di-(8CI); (Dichloro-phosphono-methyl)-phosphonic acid; (Dichloromethylene)bisphosphonic acid; (Dichloromethylene)diphosphonic acid; (Lip-C); (dichloromethanediyl)bis(phosphonic acid)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypocalcemic Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
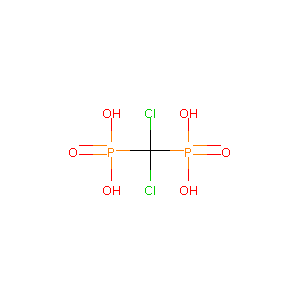 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 244.89 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
