Details of the Drug
General Information of Drug (ID: DMBP5N3)
| Drug Name |
Cantharidin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
DSSTox_CID_21752; DSSTox_RID_79835; DSSTox_GSID_41752; CAS-56-25-7; NCGC00016247-01; Spectrum_001114; SpecPlus_000537; Spectrum4_000920; Spectrum2_000630; Prestwick0_000885; Spectrum3_000621; Prestwick1_000885; Spectrum5_001618; Prestwick2_000885; KBioSS_001594; KBioGR_001420; BSPBio_002182; MLS002153505; SCHEMBL152261; DivK1c_006633; SPBio_002889; SPBio_000600; CHEMBL299846; DTXSID7041752; KBio2_006730; KBio3_001682; KBio2_004162; KBio2_001594; KBio1_001577; MolPort-003-665-556; HMS1570B12; Tox21_110326; CCG-39927; Tox21_110326_1
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
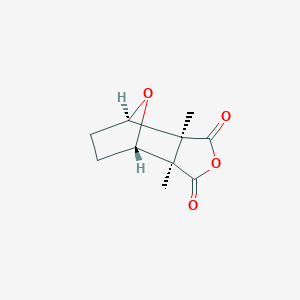 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 196.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
