Details of the Drug
General Information of Drug (ID: DMBZIVP)
| Drug Name |
Tubocurarine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Amelizol; Delacurarine; Jexin; Tubadil; Tubaine; Tubarine; Tubocurarin; Tubocurarinum; Chlorure de tubocurarine; Cloruro de tubocurarina; Dextrotubocurarine chloride; Intocostrine T; Isoquinoline alkaloid; Tubocurarina cloruro; Tubocurarina cloruro [DCIT]; Tubocurarine chloride; Tubocurarine hydrochloride; Tubocurarini chloridum; Chlorure de tubocurarine [INN-French]; Cloruro de tubocurarina [INN-Spanish]; Curarin-haf; D-Paracurarine chloride; D-Tubocurarine; D-Tubocurarine chloride; D-Tubocurarine dichloride; D-Tubocurarine hydrochloride; Delacurarine (TN); Jex (TN); Metubine (TN); Tubaine (TN); Tubarine (TN); Tubocurarine chloride (INN); Tubocurarine chloride (TN); Tubocurarine chloride (anhydrous); Tubocurarini chloridum [INN-Latin]; Tubocurarinum (TN); D-(+)-Tubocurarine chloride; Tubocurarine, chloride, hydrochloride, (+)-(8CI); D-7',12'-Dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyltubocuraranium chloride; Tubocuraranium, 7',12'-dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyl-, chloride, hydrochloride; (+)-Tubocurarine; (+)-Tubocurarine chloride; (+)-Tubocurarine chloride hydrochloride; 2,2',2'-trimethyl-6,6'-bis(methyloxy)tubocuraran-2,2'-diium-7',12'-diol dichloride; 7',12'-Dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyltubocuraranium; 7',12'-dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyltubocuraran-2'-ium
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Neuromuscular Nondepolarizing Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
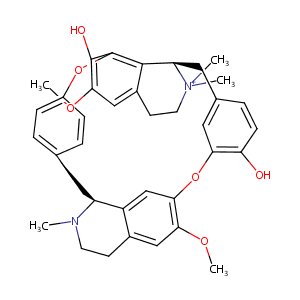 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 609.7 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Tubocurarine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
