Details of the Drug
General Information of Drug (ID: DMDJMA3)
| Drug Name |
Rizatriptan
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Risatriptan; Rizatriptanum; Rizatriptan benzoat; MK 462 free base; Maxalt (TN); Rizaliv (TN); Rizalt (TN); Rizatriptan (INN); Rizatriptan [INN:BAN]; N,N-Dimethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1H-indole-3-ethanamine; N,N-dimethyl-2-[5-(1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethanamine; N,N-Dimethyl-2-[5-(1H-1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethylamine; N,N-dimethyl-2-[5-(1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]-ethanamine; N,N-dimethyl-2-[5-(1H-1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethanamine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiinflammatory Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
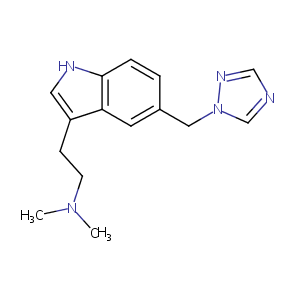 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 269.34 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Rizatriptan (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Clinical pipeline report, company report or official report of Intelgenx. | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | An introduction to migraine: from ancient treatment to functional pharmacology and antimigraine therapy. Proc West Pharmacol Soc. 2002;45:199-210. | ||||
| 4 | OCT1 mediates hepatic uptake of sumatriptan and loss-of-function OCT1 polymorphisms affect sumatriptan pharmacokinetics. Clin Pharmacol Ther. 2016 Jun;99(6):633-41. | ||||
| 5 | G protein beta3 polymorphism and triptan response in cluster headache. Clin Pharmacol Ther. 2007 Oct;82(4):396-401. doi: 10.1038/sj.clpt.6100159. Epub 2007 Mar 14. | ||||
| 6 | Chan BSH, Graudins A, Whyte IM, Dawson AH, Braitberg G, Duggin GG "Serotonin syndrome resulting from drug interactions." Med J Aust 169 (1998): 523-5. [PMID: 9861909] | ||||
| 7 | Vizcaychipi MP, Walker S, Palazzo M "Serotonin syndrome triggered by tramadol." Br J Anaesth 99 (2007): 919. [PMID: 18006535] | ||||
| 8 | Achamallah NS "Visual hallucinations after combining fluoxetine and dextromethorphan ." Am J Psychiatry 149 (1992): 1406. [PMID: 1530079] | ||||
| 9 | Product Information. Northera (droxidopa). Chelsea Therapeutics Inc, Charlotte, NC. | ||||
| 10 | Ciraulo DA, Shader RI "Fluoxetine drug-drug interactions: I. Antidepressants and antipsychotics." J Clin Psychopharmacol 10 (1990): 48-50. [PMID: 1968472] | ||||
| 11 | Alvine G, Black DW, Tsuang D "Case of delirium secondary to phenelzine/L-tryptophan combination." J Clin Psychiatry 51 (1990): 311. [PMID: 2365671] | ||||
| 12 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 13 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
