Details of the Drug
General Information of Drug (ID: DMIK367)
| Drug Name |
Procarbazine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ibenzmethyzin; Ibenzmethyzine; MBH; MIH; Natulan; Natulanar; PCX; Procarbazin; Procarbazina; Procarbazinum; Procarbazin [German]; Procarbazine Monohydrochloride; CB 400-497; Indicarb (TN); Matulane (TN); Natulan (TN); Procarbazina [INN-Spanish]; Procarbazine (INN); Procarbazine [INN:BAN]; Procarbazinum [INN-Latin];Ro 4-6467; SRI-10847; Ro 4-6467/1; N-4-Isopropylcarbamoylbenzyl-N'-methylhydrazine; N-Isopropyl-4-[(2-methylhydrazino)methyl]benzamide; P-(2-Methylhydrazinomethyl)-N-isopropylbenzamide; N-Isopropyl-p-(2-methylhydrazinomethyl)-benzamide; P-(N'-Methylhydrazinomethyl)-N-isopropylbenzamide; N-(1-Methylethyl)-4-[(2-methylhydrazino)methyl]benzamide; N-Isopropyl-alpha-(2-methylhydrazino)-p-toluamide; N-(1-Methylethyl)-4-((2-methylhydrazino)methyl)benzamide; Benzamide, N-(1-methylethyl)-4-((2-methylhydrazino)methyl)-(9CI); 1-Methyl-2-(p-(isopropylcarbamoyl)benzyl)hydrazine; 2-(p-Isopropylcarbamoylbenzyl)-1-methylhydrazine; 4-((2-Methylhydrazino)methyl)-N-isopropylbenzamide; 4-[(2-methylhydrazinyl)methyl]-N-propan-2-ylbenzamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
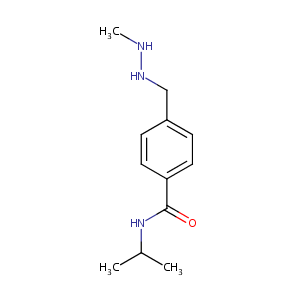 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 221.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Procarbazine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
| DIG |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmaceutical Formulation |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7278). | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 4 | Brca2/Xrcc2 dependent HR, but not NHEJ, is required for protection against O(6)-methylguanine triggered apoptosis, DSBs and chromosomal aberrations... DNA Repair (Amst). 2009 Jan 1;8(1):72-86. | ||||
| 5 | In vitro and in vivo evidence for the formation of methyl radical from procarbazine: a spin-trapping study. Carcinogenesis. 1992 May;13(5):799-805. | ||||
| 6 | Tumour cytochrome P450 and drug activation. Curr Pharm Des. 2002;8(15):1335-47. | ||||
| 7 | Canadian Pharmacists Association. | ||||
| 8 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 9 | Boakes AJ, Laurence DR, Teoh PC, Barar FS, Benedikter LT, Prichard BN "Interactions between sympathomimetic amines and antidepressant agents in man." Br Med J 1 (1973): 311-5. [PMID: 4685619] | ||||
| 10 | Adverse effects and complications of treatment with beta-adrenergic agonist drugs. Committee on drugs, the American Academy of Allergy and Immunology. J Allergy Clin Immunol 75 (1985): 443-9. [PMID: 2858503] | ||||
| 11 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 12 | Product Information. Northera (droxidopa). Chelsea Therapeutics Inc, Charlotte, NC. | ||||
| 13 | Johnson EJ, MacGowan AP, Potter MN, et al "Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy." J Antimicrob Chemother 25 (1990): 837-42. [PMID: 2373666] | ||||
| 14 | Alvine G, Black DW, Tsuang D "Case of delirium secondary to phenelzine/L-tryptophan combination." J Clin Psychiatry 51 (1990): 311. [PMID: 2365671] | ||||
| 15 | Bazire SR "Sudden death associated with switching monoamine oxidase inhibitors." Drug Intell Clin Pharm 20 (1986): 954-6. [PMID: 3816543] | ||||
| 16 | Beasley CM Jr, Masica DN, Heiligenstein JH, Wheadon DE, Zerbe RL "Possible monoamine oxidase inhibitor-serotonin uptake inhibitor interaction: fluoxetine clinical data and preclinical findings." J Clin Psychopharmacol 13 (1993): 312-20. [PMID: 8227489] | ||||
| 17 | Boyer EW, Shannon M "The serotonin syndrome." N Engl J Med 352 (2005): 1112-20. [PMID: 15784664] | ||||
| 18 | Graber MA, Hoehns TB, Perry PJ "Sertraline-phenelzine drug interaction: a serotonin syndrome reaction." Ann Pharmacother 28 (1994): 732-5. [PMID: 7919561] | ||||
| 19 | Goldberg LI "Monoamine oxidase inhibitors: adverse reactions and possible mechanisms." JAMA 190 (1964): 456-62. [PMID: 14197995] | ||||
| 20 | Product Information. Austedo (deutetrabenazine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 21 | Darcy PF, Griffin JP "Interactions with drugs used in the treatment of depressive illness." Adverse Drug React Toxicol Rev 14 (1995): 211-31. [PMID: 8845455] | ||||
| 22 | Cusson JR, Goldenberg E, Larochelle P "Effect of a novel monoamine-oxidase inhibitor, moclobemide on the sensitivity to intravenous tyramine and norepinephrine in humans." J Clin Pharmacol 31 (1991): 462-7. [PMID: 2050833] | ||||
| 23 | Product Information. Arava (leflunomide). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 24 | Product Information. Prolia (denosumab). Amgen USA, Thousand Oaks, CA. | ||||
| 25 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 26 | Product Information. Vumerity (diroximel fumarate). Alkermes, Inc, Cambridge, MA. | ||||
| 27 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 28 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 29 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 30 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 31 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 32 | Filibeck DJ, Grimm D, Forman WB, Leidner BA "Metoclopramide-induced hypertensive crisis." Clin Pharm 3 (1984): 548-9. [PMID: 6541544] | ||||
| 33 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 34 | Boakes AJ, Laurence DR, Teoh PC, Barar FS, Benedikter LT, Pritchard BN "Interactions between sympathomimetic amines and antidepressant agents in man." Br Med J 1 (1973): 311-5. [PMID: 4685619] | ||||
| 35 | Product Information. Arcalyst (rilonacept). Regeneron Pharmaceuticals Inc, Tarrytown, NY. | ||||
| 36 | Product Information. Cimzia (certolizumab). UCB Pharma Inc, Smyrna, GA. | ||||
| 37 | CDC. Centers for Disease Control and Prevention/ "Recommendations of the advisory committtee on immunization practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence." MMWR Morb Mortal Wkly Rep 42(RR-04) (1993): 1-18. [PMID: 20300058] | ||||
