Details of the Drug
General Information of Drug (ID: DM0FB1J)
| Drug Name |
Sertraline
|
|||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Lustral; SRE; Sertralina; Sertralinum; Sertralina [Spanish]; Sertraline [Zoloft]; Sertralinum [Latin]; CP 51974; Cp 51974; Apo-Sertraline; Lustral (TN); Sertraline (INN); Sertraline (Zoloft); Sertraline [INN:BAN]; Zoloft (TN); (+)-Sertraline; (1S,4S)-4-(3,4-dichlorophenyl)-N-methyl-1,2,3,4-tetrahydronaphthalen-1-amine; (1S-cis)-1,2,3,4-Tetrahydro-4-(3,4-dichlorophenyl)-N-methyl-1-naphthalenamine; (1S-cis)-4-(3,4-Dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthalenamine
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antidepressants
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
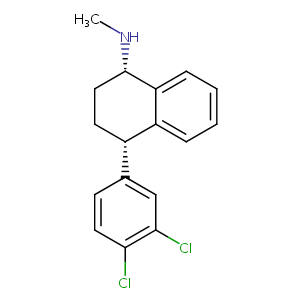 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 306.2 | ||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.8 | |||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Coronary heart disease | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA80.Z | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Sertraline
Coadministration of a Drug Treating the Disease Different from Sertraline (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Sertraline FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4798). | ||||
| 3 | Clinical pharmacokinetics of sertraline. Clin Pharmacokinet. 2002;41(15):1247-66. doi: 10.2165/00003088-200241150-00002. | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Murdoch D, McTavish D: Sertraline. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depression and obsessive-compulsive disorder. Drugs. 1992 Oct;44(4):604-24. | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 8 | Psychopharmacological treatment of dermatological patients--when simply talking does not help. J Dtsch Dermatol Ges. 2007 Dec;5(12):1101-6. | ||||
| 9 | Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012 Jan;64(1):95-109. | ||||
| 10 | New orally active anticoagulant agents for the prevention and treatment of venous thromboembolism in cancer patients. Ther Clin Risk Manag. 2014 Jun 13;10:423-36. | ||||
| 11 | PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip Rev Syst Biol Med. 2018 Jul;10(4):e1417. (ID: PA166181117) | ||||
| 12 | Some aspects of genetic polymorphism in the biotransformation of antidepressants. Therapie. 2004 Jan-Feb;59(1):5-12. | ||||
| 13 | Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab Dispos. 2005 Feb;33(2):262-70. | ||||
| 14 | Influence of CYP2B6 and CYP2C19 polymorphisms on sertraline metabolism in major depression patients. Int J Clin Pharm. 2016 Apr;38(2):388-94. | ||||
| 15 | The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Rev. 2001 Spring;7(1):1-24. | ||||
| 16 | Antidepressant drug sertraline modulates AMPK-MTOR signaling-mediated autophagy via targeting mitochondrial VDAC1 protein. Autophagy. 2021 Oct;17(10):2783-2799. doi: 10.1080/15548627.2020.1841953. Epub 2020 Nov 9. | ||||
| 17 | A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol Sci. 2005 Feb;83(2):282-92. | ||||
| 18 | Exploring binding properties of sertraline with human serum albumin: Combination of spectroscopic and molecular modeling studies. Chem Biol Interact. 2015 Dec 5;242:235-46. doi: 10.1016/j.cbi.2015.10.006. Epub 2015 Oct 22. | ||||
| 19 | Sertraline induces endoplasmic reticulum stress in hepatic cells. Toxicology. 2014 Aug 1;322:78-88. doi: 10.1016/j.tox.2014.05.007. Epub 2014 May 24. | ||||
| 20 | Sertraline, an antidepressant, induces apoptosis in hepatic cells through the mitogen-activated protein kinase pathway. Toxicol Sci. 2014 Feb;137(2):404-15. doi: 10.1093/toxsci/kft254. Epub 2013 Nov 5. | ||||
| 21 | Effects of selective serotonin reuptake inhibitors on three sex steroids in two versions of the aromatase enzyme inhibition assay and in the H295R cell assay. Toxicol In Vitro. 2015 Oct;29(7):1729-35. | ||||
| 22 | Michaelis-Menten kinetic analysis of drugs of abuse to estimate their affinity to human P-glycoprotein. Toxicol Lett. 2013 Feb 27;217(2):137-42. doi: 10.1016/j.toxlet.2012.12.012. Epub 2012 Dec 27. | ||||
| 23 | Achamallah NS "Visual hallucinations after combining fluoxetine and dextromethorphan ." Am J Psychiatry 149 (1992): 1406. [PMID: 1530079] | ||||
| 24 | Beasley CM Jr, Masica DN, Heiligenstein JH, Wheadon DE, Zerbe RL "Possible monoamine oxidase inhibitor-serotonin uptake inhibitor interaction: fluoxetine clinical data and preclinical findings." J Clin Psychopharmacol 13 (1993): 312-20. [PMID: 8227489] | ||||
| 25 | Bhatara VS, Magnus RD, Paul KL, Preskorn SH "Serotonin syndrome induced by venlafaxine and fluoxetine: a case study in polypharmacy and potential pharmacodynamic and pharmacokinetic mechanisms." Ann Pharmacother 32 (1998): 432-6. [PMID: 9562139] | ||||
| 26 | Product Information. Rexulti (brexpiprazole). Otsuka American Pharmaceuticals Inc, Rockville, MD. | ||||
| 27 | Abad S, Moachon L, Blanche P, Bavoux F, Sicard D, Salmon-Ceron D "Possible interaction between glicazide, fluconazole and sulfamethoxazole resulting in severe hypoglycaemia." Br J Clin Pharmacol 52 (2001): 456-7. [PMID: 11678792] | ||||
| 28 | Asplund K, Wiholm BE, Lithner F "Glibenclamide-associated hypoglycaemia: a report on 57 cases." Diabetologia 24 (1983): 412-7. [PMID: 6411511] | ||||
| 29 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 30 | Canadian Pharmacists Association. | ||||
| 31 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 32 | Vizcaychipi MP, Walker S, Palazzo M "Serotonin syndrome triggered by tramadol." Br J Anaesth 99 (2007): 919. [PMID: 18006535] | ||||
| 33 | Alderman CP, Moritz CK, Ben-Tovim DI "Abnormal platelet aggregation associated with fluoxetine therapy." Ann Pharmacother 26 (1992): 1517-9. [PMID: 1482806] | ||||
| 34 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 35 | Bengtsson B, Fagerstrom PO "Extrapulmonary effects of terbutaline during prolonged administration." Clin Pharmacol Ther 31 (1982): 726-32. [PMID: 7042176] | ||||
| 36 | Ball P "Quinolone-induced QT interval prolongation: a not-so-unexpected class effect." J Antimicrob Chemother 45 (2000): 557-9. [PMID: 10797074] | ||||
| 37 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 38 | Lee DO, Lee CD "Serotonin syndrome in a child associated with erythromycin and sertraline." Pharmacotherapy 19 (1999): 894-6. [PMID: 10417041] | ||||
| 39 | Belcastro V, Costa C, Striano P "Levetiracetam-associated hyponatremia." Seizure 17 (2008): 389-90. [PMID: 18584781] | ||||
| 40 | Product Information. Piqray (alpelisib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 41 | Bannister SJ, Houser VP, Hulse JD, Kisicki JC, Rasmussen JG "Evaluation of the potential for interactions of paroxetine with diazepam, cimetidine, warfarin, and digoxin." Acta Psychiatr Scand Suppl 350 (1989): 102-6. [PMID: 2530759] | ||||
| 42 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 43 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 44 | Cerner Multum, Inc. "Canadian Product Information.". | ||||
| 45 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 46 | Product Information. Austedo (deutetrabenazine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 47 | Product Information. Ingrezza (valbenazine). Neurocrine Biosciences, Inc., San Diego, CA. | ||||
| 48 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 49 | Auclair B, Berning SE, Huitt GA, Peloquin CP "Potential interaction between itraconazole and clarithromycin." Pharmacotherapy 19 (1999): 1439-44. [PMID: 10600094] | ||||
| 50 | Amchin J, Ereshefsky L, Zarycranski W, Taylor K, Albano D, Klockowski PM "Effect of venlafaxine versus fluoxetine on metabolism of dextromethorphan, a CYP2D6 probe." J Clin Pharmacol 41 (2001): 443-51. [PMID: 11304901] | ||||
| 51 | Product Information. Priftin (rifapentine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 52 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 53 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 54 | Product Information. Xalkori (crizotinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 55 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 56 | Abernethy DR, Wesche DL, Barbey JT, et al. "Stereoselective halofantrine disposition and effect: concentration-related QTc prolongation." Br J Clin Pharmacol 51 (2001): 231-7. [PMID: 11298069] | ||||
| 57 | Harper KM, Knapp DJ, Criswell HE, Breese GR "Vasopressin and alcohol: A multifaceted relationship." Psychopharmacology (Berl) 235 (2018): 3363-79. [PMID: 32936259] | ||||
| 58 | Ohnishi K, Yoshida H, Shigeno K, et al. "Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia." Ann Intern Med 133 (2000): 881-5. [PMID: 11103058] | ||||
| 59 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 60 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 61 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 62 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 63 | Alvine G, Black DW, Tsuang D "Case of delirium secondary to phenelzine/L-tryptophan combination." J Clin Psychiatry 51 (1990): 311. [PMID: 2365671] | ||||
| 64 | Chan BSH, Graudins A, Whyte IM, Dawson AH, Braitberg G, Duggin GG "Serotonin syndrome resulting from drug interactions." Med J Aust 169 (1998): 523-5. [PMID: 9861909] | ||||
| 65 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 66 | Product Information. Savella (milnacipran). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 67 | Product Information. Nuplazid (pimavanserin). Accelis Pharma, East Windsor, NJ. | ||||
| 68 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 69 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 70 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 71 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 72 | Product Information. Barhemsys (amisulpride). Acacia Pharma, Inc, Indianapolis, IN. | ||||
| 73 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 74 | Goto M, Sato M, Kitzazawa H, et.al "Papaverine-induced QT interval prolongation and ventricular fibrillation in a patient with a history of drug-induced QT prolongation." Intern Med 53 (2014): 1629-31. [PMID: 25088875] | ||||
| 75 | Alfaro CL, Lam YWF, Simpson J, Ereshefsky L "CYP2D6 status of extensive metabolizers after multiple-dose fluoxetine, fluvoxamine, paroxetine, or sertraline." J Clin Psychopharmacol 19 (1999): 155-63. [PMID: 10211917] | ||||
