Details of the Drug
General Information of Drug (ID: DM8RFNJ)
| Drug Name |
Pitolisant
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pitolisant; 362665-56-3; TIPROLISANT; BF2.649; UNII-4BC83L4PIY; 1-[3-[3-(4-chlorophenyl)propoxy]propyl]piperidine; Pitolisant (BF2.649); 1-(3-(3-(4-Chlorophenyl)propoxy)propyl)piperidine; 4BC83L4PIY; Piperidine, 1-[3-[3-(4-chlorophenyl)propoxy]propyl]-; CHEMBL462605; wakix; 1-[3-[3-(4-Chlorophenyl)propoxy]propyl]-piperidinehydrochloride; 1-{3-[3-(4-chlorophenyl)propoxy]propyl}piperidine; Tirolisant; Pitolisant (INN); tiprolisant [USAN]; Wakix (TN); Pitolisant [USAN:INN]; SCHEMBL117648; GTPL8924; CTK1B6404; HBS-101
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
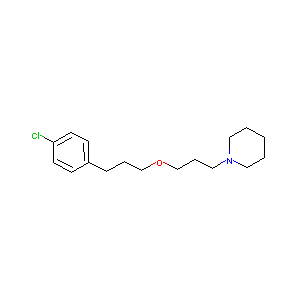 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 295.8 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Pitolisant (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019 | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT01800045) Pitolisant to Assess Weekly Frequency of Cataplexy Attacks and EDS in Narcoleptic Patients (HARMONY CTP). U.S. National Institutes of Health. | ||||
| 3 | WAKIX? (pitolisant) tablets, for oral use - FDA Label | ||||
| 4 | Wakix, INN-Pitolisant - European Medicines Agency - Europa EU | ||||
| 5 | Wakix, INN-pitolisant - European Medicines Agency - Europa EU: Assessment report | ||||
| 6 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 7 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 8 | Canadian Pharmacists Association. | ||||
| 9 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 10 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 11 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 12 | Product Information. Austedo (deutetrabenazine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 13 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 14 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 15 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 16 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 17 | Product Information. Tabrecta (capmatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 18 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 19 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 20 | Product Information. Koselugo (selumetinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 21 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 22 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 23 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 24 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 25 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 26 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 27 | Product Information. Rinvoq (upadacitinib). AbbVie US LLC, North Chicago, IL. | ||||
| 28 | Gelosa P, Castiglioni L, Tenconi M, et.al "Pharmacokinetic drug interactions of the non-vitamin K antagonist oral anticoagulants (NOACs)" Pharmacol Res 135 (2018): 60-79. [PMID: 30040996] | ||||
