Details of the Drug
General Information of Drug (ID: DME5Y64)
| Drug Name |
Tranilast
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
tranilast; 53902-12-8; Rizaben; trans-Tranilast; Tranilastum; 70806-55-2; MK 341; N-(3,4-Dimethoxycinnamoyl)anthranilic acid; Tranilastum [INN-Latin]; Tranilast [USAN:INN:JAN]; Tranpro; UNII-HVF50SMY6E; SB-252218; MK-341; Tranilast (SB 252218); 3,4-DAA; n-(3',4'-dimethoxycinnamoyl) anthranilic acid; HVF50SMY6E; MLS000028468; CHEBI:77572; C18H17NO5; Anthranilic acid, N-(3,4-dimethoxycinnamoyl)-; NZHGWWWHIYHZNX-CSKARUKUSA-N; 2-((3-(3,4-Dimethoxyphenyl)-1-oxo-2-propenyl)amino)benzoic acid; MFCD00864787; T 0318; NU-3450; Rizaben (TN); Tranilast (JAN/USAN/INN); Rizaben, SB 252218, MK 341, Tranpro ,Tranilast; N-(3',4'-dimethoxycinnamoyl) anthranilic acid; N-(3′ -Dimethoxycinnamoyl)anthranilic Acid; 2-({(2E)-3-[3,4-bis(methyloxy)phenyl]prop-2-enoyl}amino)benzoic acid; 2-[[(E)-3-(3,4-dimethoxyphenyl)prop-2-enoyl]amino]benzoic acid; 2-[[3-(3,4-Dimethoxyphenyl)-1-oxo-2-propenyl]amino] benzoic acid; Molecule 20
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
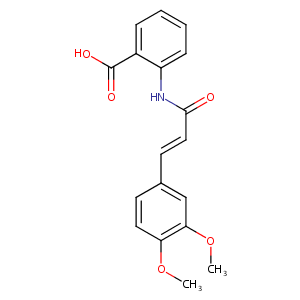 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 327.3 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergic rhinitis | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.0 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
References
