Details of the Drug
General Information of Drug (ID: DMF54ZG)
| Drug Name |
Sulpiride
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Abilit; Aiglonyl; Alimoral; Arminol; Calmoflorine; Championyl; Coolspan; Darleton; Deponerton; Desisulpid; Desmenat; Digton; Dobren; Dogmatil; Dogmatyl; Dolmatil; Dresent; Eglonil; Eglonyl; Ekilid; Enimon; Equilid; Eusulpid; Fardalan; Fidelan; Guastil; Isnamide; Kylistro; Lebopride; Levobren; Levopraid; Levosulpirida; Levosulpiridum; Lisopiride; Mariastel; Meresa; Miradol; Mirbanil; Misulvan; Neogama; Norestran; Normum; Nufarol; Omiryl; Omperan; Ozoderpin; Pontiride; Psicocen; Pyrikappl; Pyrkappl; Restful; Sernevin; Splotin; Stamonevrol; Sulp; Sulperide; Sulpirid; Sulpirida; Sulpiridum; Sulpitil; Sulpivert; Sulpor; Sulpride; Sulpyrid; Suprium; Sursumid; Synedil; Tepavil; Valirem; Zemorcon; Allphar Brand of Sulpiride; Almirall Brand of Sulpiride; Areu Brand of Sulpiride; Centrum Brand of Sulpiride; Desitin Brand of Sulpiride; Dolorgiet Brand of Sulpiride; Erempharma Brand of Sulpiride; Fumouzer Brand of Sulpiride; Hennig Brand of Sulpiride; Hexal Brand of Sulpiride; Hoechst Brand of Sulpiride; Hormosan Brand of Sulpiride; Krewel Brand of Sulpiride; Levosulpiride [INN]; Magnetic resonance imaging sulpiride; Pharmacia Brand of Sulpiride; Psicofarma Brand of Sulpiride; Rosemont Brand of Sulpiride; Sanofi Synthelabo Brand of Sulpiride; Spyfarma Brand of Sulpiride; Uriach Brand of Sulpiride; Vertigo Meresa; Vertigo neogama; RD 1403; S 8010; Bosnyl (TN); Dogmatil (TN); Dogmatyl (TN); Eglonyl (TN); Levosulpirida [INN-Spanish]; Levosulpiridum [INN-Latin]; Meresa (TN); Neuraxpharm (TN); RV-12309; Ratiopharm (TN); Sanofi-Synthelabo Brand of Sulpiride; Sulpirid (TN); Sulpirida [INN-Spanish]; Sulpiridum [INN-Latin]; Sulpiryd (TN); Vertigo-Meresa; Vertigo-neogama; R. D. 1403; R.D. 1403; Sulpiride (JP15/USAN/INN); Sulpiride [USAN:INN:BAN:JAN]; N-((1-Ethyl-2-pyrrolidinyl)methyl)-5-sulfamoyl-o-anisamide; (+/-)-Sulpiride; (RS)-(+/-)-sulpiride; (inverted question mark)-Sulpiride
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antipsychotic Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
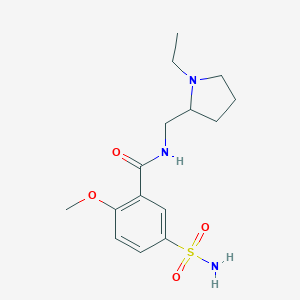 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 341.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Schizophrenia | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A20 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
