Details of the Drug
General Information of Drug (ID: DMG5OZP)
| Drug Name |
Fascaplysin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pyrido[1,2-a:3,4-b']diindol-5-ium,12,13-dihydro-13-oxo-, chloride; GNF-PF-1458; ACMC-20bu3v; AC1L2JLY; AC1Q6JA3; SCHEMBL1728912; CHEMBL602937; GTPL5969; BDBM59087; CTK4A8872; CHEBI:93765; ZINC1616841; pyrido[1,2-a:3,4-b']diindol-5-ium, 12,13-dihydro-13-oxo-; HSCI1_000331; NCGC00346951-01; CJ-26101; BRD-K13287209-003-03-2; BRD-K13287209-311-02-1; BRD-K13287209-311-01-3; BRD-K13287209-003-02-4; BRD-K13287209-003-01-6
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
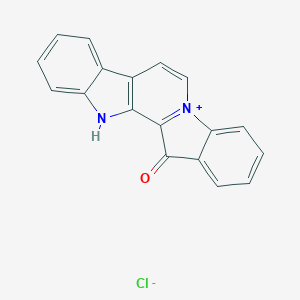 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 306.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
