Details of the Drug
General Information of Drug (ID: DMG65CV)
| Drug Name |
INCB057643
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
INCB-057643; 1820889-23-3; UNII-87TZD0JEBS; 87TZD0JEBS; 2,2,4-trimethyl-8-(6-methyl-7-oxo-6,7-dihydro-1H-pyrrolo[2,3-c]pyridin-4-yl)-6-(methylsulfonyl)-2H-benzo[b][1,4]oxazin-3(4H)-one; 2,2,4-Trimethyl-8-(6-methyl-7-oxo-6,7-dihydro-1H-pyrrolo(2,3-C)pyridin-4-yl)-6-(methylsulfonyl)-2H-benzo(b)(1,4)oxazin-3(4H)-one; CHEMBL4594412; SCHEMBL17200525; US9957268, Example 75; BDBM391587; BCP29506; EX-A1908; NSC807398; s8714; NSC-807398; BS-16298; HY-111485; AK00799038; CS-0042192; INCB057643; INCB 057643; CC1(OC2=C(N(C1=O)C)C=C(C=C2C=2C1=C(C(N(C2)C)=O)NC=C1)S(=O)(=O)C)C; 2,2,4-trimethyl-8-(6-methyl-7- oxo-6,7-dihydro-1H- pyrrolo[2,3-c]pyridin-4-yl)-6- (methylsulfonyl)-2H-1,4- benzoxazin-3(4H)-one; 2H-1,4-Benzoxazin-3(4H)-one, 8-(6,7-dihydro-6-methyl-7-oxo-1H-pyrrolo(2,3-C)pyridin-4-yl)-2,2,4-trimethyl-6-(methylsulfonyl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
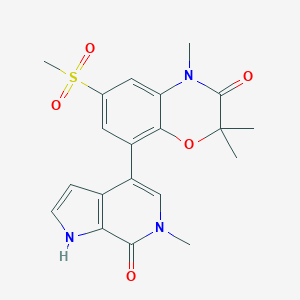 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 415.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
