Details of the Drug
General Information of Drug (ID: DMGX5Q0)
| Drug Name |
Gepirone
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
83928-76-1; 4,4-Dimethyl-1-(4-(4-(pyrimidin-2-yl)piperazin-1-yl)butyl)piperidine-2,6-dione; Gepironum [Latin]; Gepirone [INN]; Gepirona [Spanish]; UNII-JW5Y7B8Z18; BMY-13805; JW5Y7B8Z18; CHEMBL284092; Gepironum; AK115965; Gepirona; Travivo; 2,6-Piperidinedione, 4,4-dimethyl-1-(4-(4-(2-pyrimidinyl)-1-piperazinyl)butyl)-; BMY 13805; gepiron ER; MJ 13805-1; ORG-13011(Gepirone); AC1L1IK6; AC1Q6F8Q; 4,4-dimethyl-1-(4-(4-(2-pyrimidinyl)-1-piperazinyl)butyl)-2,6-piperidinedione; 3,3-dimethyl-1-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]glutarimide; 4,4-dimethyl-1-[4-(4-pyrimidin-2-ylpiperazin-1-yl)butyl]piperidine-2,6-dione
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
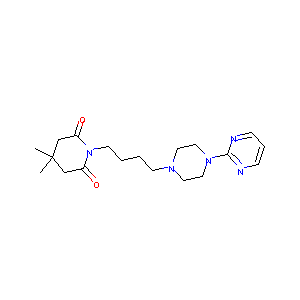 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 359.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.8 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Major depressive disorder | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A70.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
