Details of the Drug
General Information of Drug (ID: DMHAZLM)
| Drug Name |
NARINGENIN
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
naringenin; 5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one; 67604-48-2; 4',5,7-Trihydroxyflavanone; Naringenine; (+/-)-Naringenin; naringetol; 480-41-1; salipurpol; (-)-Naringenin; NARIGENIN; Salipurol; 5,7,4'-Trihydroxyflavanone; 93602-28-9; (S)-Naringenin; BE-14348A; NSC 34875; ( inverted exclamation markA)-Naringenin; CHEMBL32571; MLS000738094; MLS000028739; CHEBI:50202; Flavanone, 4',5,7-trihydroxy-; FTVWIRXFELQLPI-UHFFFAOYSA-N; NSC34875; NSC11855; MFCD00006844; SMR000059039; AK122638; NSC 11855; 4',7-Trihydroxyflavanone
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Structure |
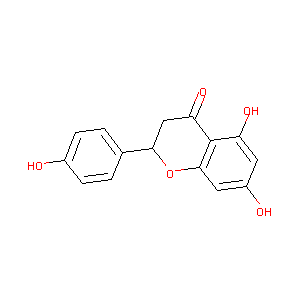 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 272.25 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | |||||
| Rotatable Bond Count (rotbonds) | 1 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | |||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
References
