Details of the Drug
General Information of Drug (ID: DMSJVAM)
| Drug Name |
Amisulpride
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aminosultopride; Amisulpiride; Amisulprida; Amisulpridum; Deniban; Socian; Solian; Amisulpride [INN]; DAN 2163; Amisulprida [INN-Spanish]; Amisulpride (INN); Amisulpridum [INN-Latin]; DAN-2163; Deniban (TN); Solian (TN); Solian, Amisulpride; SL-91.1077; 4-Amino-N-((1-ethyl-2-pyrrolidinyl)methyl)-5-(ethylsulfonyl)-2-anisamid; 4-Amino-N-((1-ethyl-2-pyrrolidinyl)methyl)-5-(ethylsulfonyl)-2-methoxybenzamide; 4-Amino-N-((1-ethyl-2-pyrrolidinyl)methyl)-5-(ethylsulfonyl)-o-anisamide; 4-Amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide; 4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide; 4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-ethylsulfonyl-2-methoxybenzamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antipsychotic Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
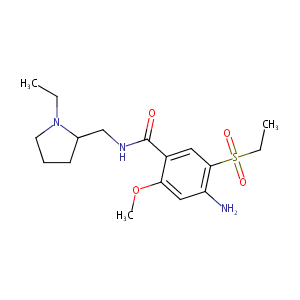 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 369.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Schizophrenia | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A20 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Amisulpride (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 963). | ||||
|---|---|---|---|---|---|
| 2 | Boison D, Yegutkin GG: Adenosine Metabolism: Emerging Concepts for Cancer Therapy. Cancer Cell. 2019 Dec 9;36(6):582-596. doi: 10.1016/j.ccell.2019.10.007. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | FDA Approved Drug Products: BARHEMSYS (amisulpride) injection, for intravenous use | ||||
| 5 | Amisulpride: a review of its use in the management of schizophrenia. Drugs. 2001;61(14):2123-50. doi: 10.2165/00003495-200161140-00014. | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Summary of Product Characteristics: Amisulpride Oral Tablets | ||||
| 8 | Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20(5):389-409. | ||||
| 9 | Identification of P-glycoprotein substrates and inhibitors among psychoactive compounds--implications for pharmacokinetics of selected substrates. J Pharm Pharmacol. 2004 Aug;56(8):967-75. | ||||
| 10 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 11 | Product Information. Barhemsys (amisulpride). Acacia Pharma, Inc, Indianapolis, IN. | ||||
| 12 | Product Information. Olinvyk (oliceridine). Trevena Inc, Chesterbrook, PA. | ||||
| 13 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 14 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 15 | Bengtsson B, Fagerstrom PO "Extrapulmonary effects of terbutaline during prolonged administration." Clin Pharmacol Ther 31 (1982): 726-32. [PMID: 7042176] | ||||
| 16 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 17 | Canadian Pharmacists Association. | ||||
| 18 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 19 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 20 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 21 | Mims RB, Scott CL, Modebe O, Bethune JE "Inhibition of L-dopa-induced growth hormone stimulation by pyridoxine and chlorpromazine." J Clin Endocrinol Metab 40 (1975): 256-9. [PMID: 1117978] | ||||
| 22 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 23 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
