Details of the Drug
General Information of Drug (ID: DMNLHAC)
| Drug Name |
Allopregnanolone
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
516-54-1; Brexanolone; Allotetrahydroprogesterone; Allopregnan-3alpha-ol-20-one; 3alpha-hydroxy-5alpha-pregnan-20-one; SAGE-547; UNII-S39XZ5QV8Y; 3-alpha,5-alpha-Pregnanolone; 3alpha-OH DHP; 3alpha,5alpha-THP; 5alpha-Pregnan-3alpha-ol-20-one; 3a-Hydroxy-5a-pregnan-20-one; 3a,5a-THP; (3alpha)-Allopregnanolone; 3-alpha,5-alpha-Tetrahydroprogesterone; BRN 3211363; S39XZ5QV8Y; 3alpha-Hydroxy-5alpha-dihydroprogesterone; 3-alpha-Hydroxy-5-alpha-pregnan-20-one; CHEMBL207538; CHEBI:50169; SGE-102
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
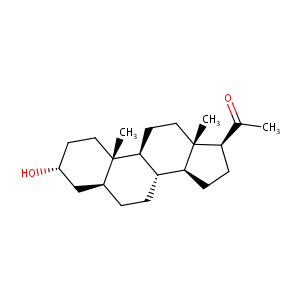 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 318.5 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.9 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Allopregnanolone (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019 May;18(5):339-357. | ||||
|---|---|---|---|---|---|
| 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 4 | Neurosteroid analogues. 10. The effect of methyl group substitution at the C-6 and C-7 positions on the GABA modulatory and anesthetic actions of (... J Med Chem. 2005 Apr 21;48(8):3051-9. | ||||
| 5 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 6 | Neurosteroid analogues. 11. Alternative ring system scaffolds: gamma-aminobutyric acid receptor modulation and anesthetic actions of benz[f]indenes. J Med Chem. 2006 Jul 27;49(15):4595-605. | ||||
| 7 | FDA Label of Brexanolone. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 8 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
