Details of the Drug
General Information of Drug (ID: DMI7FVQ)
| Drug Name |
Metyrapone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
MYT; Mepyrapone; Metapirone; Metapyron; Methapyrapone; Methbipyranone; Methopirapone; Methopyrapone; Methopyrinine; Methopyrone; Metirapona; Metopiron; Metopirone; Metopyrone; Metroprione; Metyrapon; Metyraponum; Alliance Brand of Metyrapone; Metyrapone Alliance Brand; Metyrapone Novartis Brand; Novartis Brand of Metyrapone; Su 4885; METOPIRONE (TN); Metirapona [INN-Spanish]; Metopirone (TN); Metyraponum [INN-Latin]; Su-4885; Metyrapone (JP15/USP/INN); Metyrapone [USAN:INN:BAN:JAN]; 1,2-Di-3-pyridyl-2-methyl-1-propanone; 2-Methyl-1,2-bis(3-pyridyl)-1-propanone; 2-Methyl-1,2-di-3-pyridinyl-1-propanone; 2-Methyl-1,2-di-3-pyridyl-1-propanone; 2-methyl-1,2-dipyridin-3-yl-propan-1-one; 2-methyl-1,2-dipyridin-3-ylpropan-1-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antimetabolites
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
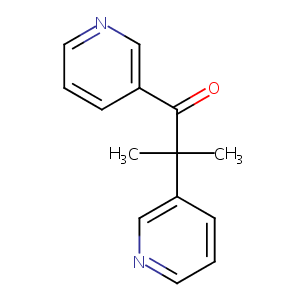 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 226.27 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Cushing disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A70 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5224). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Effects of 3-MeSO2-DDE and some CYP inhibitors on glucocorticoid steroidogenesis in the H295R human adrenocortical carcinoma cell line. Toxicol In Vitro. 2002 Apr;16(2):113-21. | ||||
| 5 | In the search for specific inhibitors of human 11beta-hydroxysteroid-dehydrogenases (11beta-HSDs): chenodeoxycholic acid selectively inhibits 11beta-HSD-I. Eur J Endocrinol. 2000 Feb;142(2):200-7. | ||||
| 6 | The H295R system for evaluation of endocrine-disrupting effects. Ecotoxicol Environ Saf. 2006 Nov;65(3):293-305. | ||||
| 7 | Induction of ABCC3 (MRP3) by pregnane X receptor activators. Drug Metab Dispos. 2003 Nov;31(11):1296-9. doi: 10.1124/dmd.31.11.1296. | ||||
| 8 | A reporter gene assay to assess the molecular mechanisms of xenobiotic-dependent induction of the human CYP3A4 gene in vitro. Xenobiotica. 1999 Mar;29(3):269-79. | ||||
| 9 | Biochemical properties of human dehydrogenase/reductase (SDR family) member 7. Chem Biol Interact. 2014 Jan 25;207:52-7. doi: 10.1016/j.cbi.2013.11.003. Epub 2013 Nov 16. | ||||
| 10 | In vivo and mechanistic evidence of nuclear receptor CAR induction by artemisinin. Eur J Clin Invest. 2006 Sep;36(9):647-53. doi: 10.1111/j.1365-2362.2006.01700.x. | ||||
| 11 | Mineralocorticoid receptor function in posttraumatic stress disorder after pretreatment with metyrapone. Biol Psychiatry. 2006 Oct 1;60(7):784-7. doi: 10.1016/j.biopsych.2006.01.014. Epub 2006 Mar 29. | ||||
