Details of the Drug
General Information of Drug (ID: DMJ6H1Z)
| Drug Name |
Quinestrol
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
EECPE; Eston; Estrovis; Estrovister; Plestrovis; Quilea; Quinestrolo; Quinestrolum; Quinestrolo [DCIT]; Estrovis 4000; W 3566; Estrovis (TN); Ethinyl Estradiol 3-Cyclopentyl Ether; Qui-Lea; Quinestrolum [INN-Latin]; W-3566; Quinestrol (USAN/INN); Quinestrol [USAN:INN:BAN]; Estradiol-17-beta 3-cyclopentyl ether; (8R,9S,13S,14S,17R)-3-cyclopentyloxy-17-ethynyl-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-ol; 17-alpha-Ethinylestradiol 3-cyclopentyl ether; 17ALPHA-ETHYLNYL-1,3,5[10]ESTRATRIENE-3,17BETA-DIOL 3-CYCLOPENTYL ETHER; 17alpha-Ethynyl-1,3,5(10)-estratriene-3,17beta-diol 3-cyclopentyl ether; 17alpha-Ethynylestradiol 3-cyclopentyl ether; 3-(Cyclopentyloxy)-19-nor-17-alpha-pregna-1,3,5(10)-trien-20-yn-17-ol; 3-(Cyclopentyloxy)-19-nor-17alpha-pregna-1,3,5(10)-trien-20-yn-17-ol; 3-(cyclopentyloxy)-17beta-ethynylestra-1,3,5(10)-trien-17-ol; 3-Cyclopentyloxy-17alpha-ethynylestra-1,3,5(10)-trien-17beta-ol
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
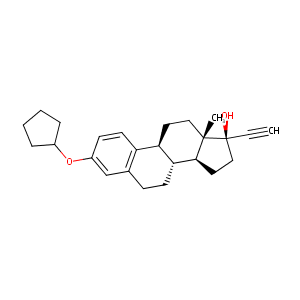 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 364.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Quinestrol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
