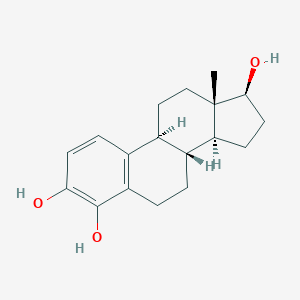
| Synonyms |
4-Hydroxy-17-beta-estradiol; 4-Hydroxyestradiol-17beta; 4-OH-Estradiol; 4-hydroxy-estradiol; 4-HYDROXYESTRADIOL; 4-hydroxyestradiol-17 beta; 4OHE2; 5976-61-4; C3ZO03450E; CHEBI:62845; MLS000069567; SMR000058747; UNII-C3ZO03450E; estra-1,3,5(10)-triene-2,4,17beta-triol; 4-Hydroxyestradiol; (8R,9S,13S,14S,17S)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,4,17-triol; 1,3,5(10)-Estratriene-3,4,17beta-triol; 1,3,5[10]-ESTRATRIENE-3,4,17BETA-TRIOL; 3,4,17beta-estriol; 3,4,17beta-trihydroxy-1,3,5[10]-estratriene
|
| Chemical Identifiers |
- Formula
- C18H24O3
- IUPAC Name
(8R,9S,13S,14S,17S)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,4,17-triol - Canonical SMILES
-
CC12CCC3C(C1CCC2O)CCC4=C3C=CC(=C4O)O
- InChI
-
QOZFCKXEVSGWGS-ZHIYBZGJSA-N
- InChIKey
-
1S/C18H24O3/c1-18-9-8-11-10-4-6-15(19)17(21)13(10)3-2-12(11)14(18)5-7-16(18)20/h4,6,11-12,14,16,19-21H,2-3,5,7-9H2,1H3/t11-,12-,14+,16+,18+/m1/s1
|