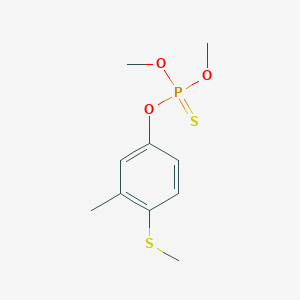
| DOT Name |
DOT ID |
UniProt ID |
Interaction |
REF |
|
Acetylcholinesterase (ACHE)
|
OT2H8HG6 |
ACES_HUMAN
|
Gene/Protein Processing |
[2] |
|
Breast cancer type 1 susceptibility protein (BRCA1)
|
OT5BN6VH |
BRCA1_HUMAN
|
Gene/Protein Processing |
[3] |
|
Cellular tumor antigen p53 (TP53)
|
OTIE1VH3 |
P53_HUMAN
|
Gene/Protein Processing |
[4] |
|
Cholinesterase (BCHE)
|
OTOH3WQ9 |
CHLE_HUMAN
|
Gene/Protein Processing |
[5] |
|
Cytochrome P450 3A4 (CYP3A4)
|
OTQGYY83 |
CP3A4_HUMAN
|
Regulation of Drug Effects |
[6] |
|
Cytochrome P450 3A5 (CYP3A5)
|
OTSXFBXB |
CP3A5_HUMAN
|
Regulation of Drug Effects |
[6] |
|
Cytochrome P450 3A7 (CYP3A7)
|
OTTCDHHM |
CP3A7_HUMAN
|
Regulation of Drug Effects |
[6] |
|
DNA repair protein RAD51 homolog 1 (RAD51)
|
OTNVWGC1 |
RAD51_HUMAN
|
Gene/Protein Processing |
[3] |
|
DNA repair protein XRCC2
|
OTJB2PV4 |
XRCC2_HUMAN
|
Gene/Protein Processing |
[3] |
|
E3 ubiquitin-protein ligase RAD18 (RAD18)
|
OTQ9PLNT |
RAD18_HUMAN
|
Gene/Protein Processing |
[3] |
|
| ------------------------------------------------------------------------------------ |
|
|
|
|