Details of the Drug
General Information of Drug (ID: DMKJ8LB)
| Drug Name |
Josamycin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Josacine; Josamicina; Josamycine; Josamycinum; EN 141; Kitasamycin A3; Leucomycin A3; Turimycin A5; Antibiotic yl-704 A3; Iosalide (TN); Josacine (TN); Josalid (TN); Josamicina [INN-Spanish]; Josamina (TN); Josamy (TN); Josamycin (TN); Josamycine [INN-French]; Josamycinum [INN-Latin]; Wilprafen (TN); Yl-704 A3; Josamycin [USAN:INN:JAN]; Josamycin (JP15/USAN/INN); Dro-2H-pyran-3-yl 3-methylbutanoate; Leucomycin V, 3-acetate 4B-(3-methylbutanoate); Leucomycin V, 3-acetate 4(sup B)-(3-methylbutanoate); Leucomycin V,3-acetate 4(sup beta)-(3-methylbutanoate)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
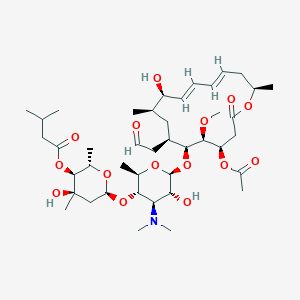 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 828 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 14 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 16 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
