Details of the Drug
General Information of Drug (ID: DMLFSTR)
| Drug Name |
Ticrynafen
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ticrynafen; Tienilic acid; 40180-04-9; Thienylic acid; Selacryn; Diflurex; Ticrex; Acido tienilico; Acide tienilique; Acidum tienilicum; Tienilico acido; Tienilico acido [Spanish]; Ticrynafen [USAN]; Acido tienilico [INN-Spanish]; Acide tienilique [INN-French]; Acidum tienilicum [INN-Latin]; ANP 3624; C13H8Cl2O4S; SKF 62698; UNII-HC95205SY4; 2-(2,3-dichloro-4-(thiophene-2-carbonyl)phenoxy)acetic acid; (2,3-Dichloro-4-(2-thenoyl)phenoxy)acetic acid; CCRIS 5489; 4-(2-Thienylketo)-2,3-dichlorophenoxyacetic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
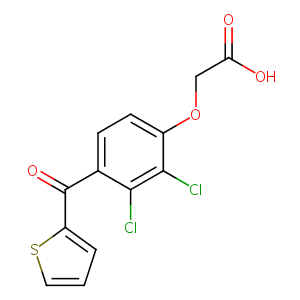 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 331.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
