Details of the Drug
General Information of Drug (ID: DMM0HR1)
| Drug Name |
Pargyline
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Benzylmethylpropargylamine; Benzylmethylpropynylamine; Eudatin; Eutron; Methylbenzylpropynylamine; Paragyline; Pargilina; Pargylamine; Pargylin; Pargylinum; Supirdyl; A 19120; MO 911; Pargilina [INN-Spanish]; Pargyline (INN); Pargyline [INN:BAN]; Pargylinum [INN-Latin]; Benzyl-methyl-2-propinylamin; Benzyl-methyl-2-propinylamin [Czech]; N-Methyl-N-benzylpropynylamine; N-Methyl-N-propargylbenzylamine; N-Methyl-N-2-propynylbenzylamine; N-Benzyl-N-methyl-2-propynylamine; N-Benzyl-N-methyl-2-propyn-1; N-Benzyl-N-methyl-2-propyn-1-amine; N-benzyl-N-methylprop-2-yn-1-amine; N-methyl-N-(phenylmethyl)prop-2-yn-1-amine
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
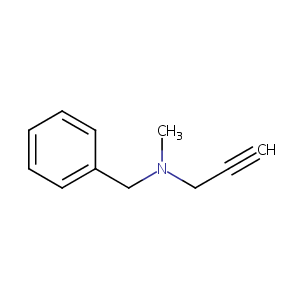 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 159.23 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hypertension | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA00-BA04 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
