Details of the Drug
General Information of Drug (ID: DMMAWY1)
| Drug Name |
Adefovir Dipivoxil
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Adefovirdipivoxl; Hepsera; Preveon; YouHeDing; Adefovir depivoxil; Adefovir pivoxil; GS 0840; GS 840;Piv2PMEA; Adefovir dipivoxil (USAN); Adefovir pivoxil (JAN); Bis(pom)PMEA; Bis-POM PMEA; GS-0840; GS-840; Hepsera (TM); Hepsera (TN); Preveon (TN); Bis(POM)-PMEA; Bis-POM PMEA, Adefovir Dipivoxil; Bis-POM PMEA, Preveon, Hepsera, Adefovir Dipivoxil; Bis(pivaloyloxymethyl)-9-(2-phosphonylmethoxyethyl)adenine; [2-(6-aminopurin-9-yl)ethoxymethyl-(2,2-dimethylpropanoyloxymethoxy)phosphoryl]oxymethyl 2,2-dimethylpropanoate; Propanoic acid,2,2-dimethyl-(((2-(6-amino-9H-purin-9-yl)ehtoxy)mehtyl)phosphinyldiene)bis(oxymehtylene)ester; Propanoic acid, 2,2-dimethyl-, (((2-(6-amino-9H-purin-9-yl)ethoxy)methyl)phosphinylidene)bis(oxymethylene) ester; (((2-(6-Amino-9H-purin-9-yl)ethoxy)methyl)phosphinylidene)bis(oxymethylene) 2,2-dimethylpropanoate; ((2-(6-Amino-9H-purin-9-yl)ethoxy)methyl)phosphonic acid, diester with hydroxymethyl pivalate; 9-(2-((-Bis((pivaloyloxy)methoxy)phosphinyl)methoxy)ethyl)adenine; 9-(2-((Bis((pivaloyloxy)methoxy)phosphinyl)methoxy)ethyl)adenine; ADV
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agents
|
||||||||||||||||||||||
| Affected Organisms |
Hepatitis B virus
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
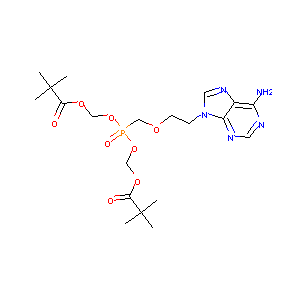 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 501.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 15 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Telbivudine: a new option for the treatment of chronic hepatitis B. Expert Opin Biol Ther. 2007 May;7(5):751-61. | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 4 | Scientific discussion of Hepsera. | ||||
| 5 | Transcriptomics hit the target: monitoring of ligand-activated and stress response pathways for chemical testing. Toxicol In Vitro. 2015 Dec 25;30(1 Pt A):7-18. | ||||
