Details of the Drug
General Information of Drug (ID: DMMJZYC)
| Drug Name |
CH-223191
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
301326-22-7; CH 223191; CH223191; UNII-HYE7315Z4C; HYE7315Z4C; CHEMBL1743245; AhR Antagonist; 2-methyl-N-[2-methyl-4-[(2-methylphenyl)diazenyl]phenyl]pyrazole-3-carboxamide; 1-Methyl-N-[2-methyl-4-[2-(2-methylphenyl)diazenyl]phenyl-1H-pyrazole-5-carboxamide; 2-methyl-N-[2-methyl-4-(2-methylphenyl)azophenyl]-3-pyrazolecarboxamide; (E)-1-methyl-N-(2-methyl-4-(o-tolyldiazenyl)phenyl)-1H-pyrazole-5-carboxamide; 1-methyl-N-{2-methyl-4-[(E)-(2-methylphenyl)diazenyl]phenyl}-1H-pyrazole-5-carboxamide; 2-Methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide; CBDivE_007629; SCHEMBL363547; DTXSID5058698; AOB1977; EX-A771; SYN5007; CHEBI:125508; HMS3412G22; HMS3676G22; HMS3750E07; BCP12814; ZINC4762981; BDBM50525572; MFCD00377884; s7711; SBB081907; STK837883; 2-Methyl-2H-pyrazole-3-carboxylic acid-(2-methyl-4-o-tolyl-azophenyl)-amide; AKOS000629906; AKOS026750492; ZINC100550218; ZINC254429865; CCG-267843; CS-3905; MCULE-3225752823; 1H-Pyrazole-5-carboxamide, 1-methyl-N-(2-methyl-4-((2-methylphenyl)azo)phenyl)-; 1H-Pyrazole-5-carboxamide, 1-methyl-N-(2-methyl-4-(2-(2-methylphenyl)diazenyl)phenyl)-; AC-32878; AS-16374; HY-12684; QC-11824; FT-0696668; X3558; J-017795; BRD-K22314899-001-01-6; Q27216129; Q27280162; 1-methyl-N-{2-methyl-4-[2-(2-methylphenyl)diazen-1-yl]phenyl}-1H-pyrazole-5-carboxamide; N-{2-methyl-4-[(2-methylphenyl)diazenyl]phenyl}(1-methylpyrazol-5-yl)carboxami de
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
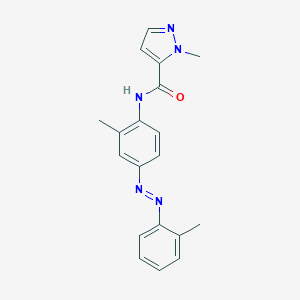 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 333.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
