Details of the Drug
General Information of Drug (ID: DMVPLNC)
| Drug Name |
Ingrezza
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Valbenazine; 1025504-45-3; UNII-54K37P50KH; Valbenazine free base; NBI 98854; 54K37P50KH; 1025504-45-3 (free base); (2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl L-valinate; L-Valine, (2R,3R,11bR)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo[a]quinolizin-2-yl ester; Valbenazine [USAN:INN]; L-Valine, (2R,3R,11bR)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo(a)quinolizin-2-yl ester; ValbenazineNBI-98854; Valbenazine (USAN/INN); GTPL8694; CHEMBL2364639; SCHEMBL15932979; EX-A2002; MFCD28963976; ZINC43195697; CS-5908; DB11915; SB17456; NCGC00522306-02; [(2R,3R,11bR)-9,10-dimethoxy-3-(2-methylpropyl)-2,3,4,6,7,11b-hexahydro-1H-benzo[a]quinolizin-2-yl] (2S)-2-amino-3-methylbutanoate; AC-30929; AS-35294; HY-16771; D10675; NBI-98854;NBI98854;NBI 98854; Q27089118; (2R,3R,11bR)-9,10-Dimethoxy-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydro-2H- benzo(a)quinolizin-2-yl L-valinate; (S)-2-amino-3-methyl-butyric acid (2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl ester; [(2R,3R,11bR)-9,10-dimethoxy-3-(2-methylpropyl)-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl] (2S)-2-amino-3-methylbutanoate; Valine 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo(a)quinolizin-2-yl ester
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
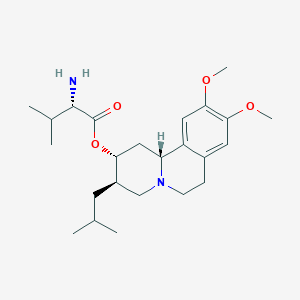 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 418.6 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Tardive dyskinesia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A02.10 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ingrezza (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | ClinicalTrials.gov (NCT03698331) The Potential for Clinical Dependence and Withdrawal Symptoms Associated With Valbenazine. U.S. National Institutes of Health. | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT03732534) Rollover Study for Continuing NBI-98854 Administration in Pediatric Subjects With Tourette Syndrome. U.S. National Institutes of Health. | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | VMAT2 Inhibitors and the Path to Ingrezza (Valbenazine). Prog Med Chem. 2018;57(1):87-111. | ||||
| 5 | Valbenazine in the treatment of tardive dyskinesia. Neurodegener Dis Manag. 2019 Apr;9(2):73-81. doi: 10.2217/nmt-2019-0001. Epub 2019 Feb 6. | ||||
| 6 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 7 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 8 | Product Information. Ingrezza (valbenazine). Neurocrine Biosciences, Inc., San Diego, CA. | ||||
| 9 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 10 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 11 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 12 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 13 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 14 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 15 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 16 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 17 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 18 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 19 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 20 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 21 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 22 | Product Information. Bevyxxa (betrixaban). Portola Pharmaceuticals, South San Francisco, CA. | ||||
