Details of the Drug
General Information of Drug (ID: DMNZWL7)
| Drug Name |
Moclobemide
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Arima; Aurorex; Aurorix; Deprenorm; Feraken; Manerix; Moclaime; Moclamide; Moclamine; Moclix; Moclobamide; Moclobemid; Moclobemida; Moclobemidum; Moclobeta; Moclodura; Moclonorm; NovoMoclobemide; NuMoclobemide; Rimoc; Alphapharm Brand of Moclobemide; Alpharma Brand of Moclobemide; Apo Moclobemide; Apotex Brand of Moclobemide; Azupharma Brand of Moclobemide; BC Brand of Moclobemide; Betapharm Brand of Moclobemide; Bull Brand of Moclobemide; Chem mart Brand of Moclobemide; Chem mart Moclobemide; Ct Arzneimittel Brand of Moclobemide; DBL Moclobemide; Esparma Brand of Moclobemide; Faulding Brand of Moclobemide; GenRX Moclobemide; Healthsense Brand of Moclobemide; Healthsense Moclobemide; Hexal Brand of Moclobemide; Hoffmann La Roche Brand of Moclobemide; Kendrick Brand of Moclobemide; Merck dura Brand of Moclobemide; Moclobemid AZU; Moclobemid Puren; Moclobemid Stada; Moclobemid ratiopharm; Moclobemid von ct; Moclobemide Alphapharm Brand; Moclobemide Alpharma Brand; Moclobemide Apotex Brand; Moclobemide Azupharma Brand; Moclobemide BC Brand; Moclobemide Bull Brand; Moclobemide Faulding Brand; Moclobemide Healthsense Brand; Moclobemide Hexal Brand; Moclobemide Kendrick Brand; Moclobemide Novopharm Brand; Moclobemide Pharmascience Brand; Moclobemide Roche Brand; Moclobemide Stadapharm Brand; Moclobemide Temmler Brand; Moclobemide betapharm Brand; Moclobemide esparma Brand; Moclobemide ratiopharm Brand; Novo Moclobemide; Novopharm Brand of Moclobemide; Nu Moclobemide; Nu Pharm Brand of Moclobemide; PMS Moclobemide; Pharmascience Brand of Moclobemide; Ratiopharm Brand of Moclobemide; Roche Brand of Moclobemide; Stadapharm Brand of Moclobemide; Temmler Brand of Moclobemide; Terry White Chemists Brand of Moclobemide; Terry White Chemists Moclobemide; CBMicro_048319; Moclobemid 1A Pharma; Moclobemid1A Pharma; Moclobemide 1A Brand; Ro 11 1163; AZU, Moclobemid; Apo-Moclobemide; Aurorix (TN); Ct-Arzneimittel Brand of Moclobemide; Hoffmann-La Roche Brand of Moclobemide; Manerix (TN); Moclobemid-1A Pharma; Moclobemid-Puren; Moclobemid-ratiopharm; Moclobemida [INN-Spanish]; Moclobemide Nu-Pharm Brand; Moclobemide ct-Arzneimittel Brand; Moclobemide, Chem mart; Moclobemide, DBL; Moclobemide, GenRX; Moclobemide, Healthsense; Moclobemidum [INN-Latin]; Novo-Moclobemide; Nu-Moclobemide; Nu-Pharm Brand of Moclobemide; PMS-Moclobemide; Ro 11-1163; Stada, Moclobemid; Von ct, Moclobemid; GNF-PF-695; Moclobemide (USAN/INN); Moclobemide [USAN:BAN:INN]; Moclobemide [USAN:INN:BAN]; Ro 11-1163/000; Ro-11-1163; P-Chloro-N-(2-morpholinoethyl)benzamide; 1A Brand of Moclobemide; 4-Chlor-N-(2-morpholinoethyl)benzamid; 4-Chloro-N-(2-(4-morpholinyl)ethyl)benzamide; 4-Chloro-N-(2-morpholin-4-yl-ethyl)-benzamide; 4-chloro-N-(2-morpholin-4-ylethyl)benzamide; 4-chloro-N-[2-(morpholin-4-yl)ethyl]benzamide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antidepressants
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
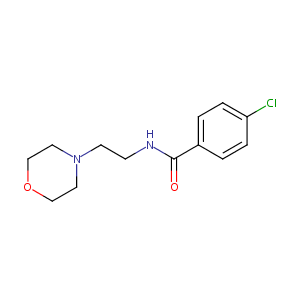 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 268.74 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Depression | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A70-6A7Z | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
References
