Details of the Drug
General Information of Drug (ID: DMOX1CU)
| Drug Name |
NCX-4016
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Nitroaspirin; Ncx 4016; NO-ASA; Nitric oxide-releasing aspirin; M-NO-ASA; M-NO-aspirin; [3-(nitrooxymethyl)phenyl] 2-acetyloxybenzoate; Benzoic acid, 2-(acetyloxy)-, 2-((nitrooxy)methyl)phenyl ester; Benzoic acid, 2-(acetyloxy)-, 3-((nitrooxy)methyl)phenyl ester; 2-((Nitrooxy)methyl)phenyl 2-(acetyloxy)benzoate; 2-Acetoxybenzoate-2-(1-nitroxymethyl)phenyl ester; 2-Acetoxybenzoic acid 3-(nitrooxymethyl)phenyl ester; 2-Acetoxybenzoic acid 3-nitrooxymethylphenyl ester; 3-((Nitrooxy)methyl)phenyl 2-(acetyloxy)benzoate; 3-(Nitroxymethyl)phenyl 2-acetoxybenzoate
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
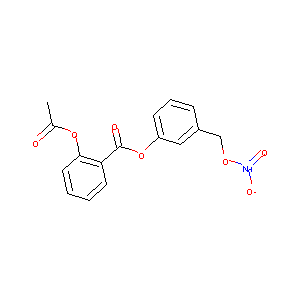 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 331.28 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
