Details of the Drug
General Information of Drug (ID: DMPI6Z0)
| Drug Name |
Zopiclone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Amoban; Amovane; Imovance; Imovane; Limovan; Optidorm; Rhovane; Siaten; Sopivan; Ximovan; Zileze; Zimoclone; Zimovane; Zopicalm; Zopicalma; Zopiclodura; Zopiclona; Zopiclonum; Zopitan; Zorclone; AbZ brand of zopiclone; Aliud brand of zopiclone; Alpharma brand of zopiclone; Aventis Pharma brand of zopiclone; Aventis brand of zopiclone; Azupharma brand of zopiclone; Betapharm brand of zopiclone; Ciclum brand of zopiclone; Clonmel brand of zopiclone; Dolorgeit brand of zopiclone; Gerard brand of zopiclone; Hexal brand of zopiclone; Hormosan brand of zopiclone; Italfarmaco brand of zopiclone; Merck dura brand of zopiclone; Neuraxpharm brand of zopiclone; Norton brand of zopiclone; Opus brand of zopiclone; Pinewood brand of zopiclone; Ratiopharm brand of zopiclone; Rhodiapharm brand of zopiclone; Stadapharm brand of zopiclone; TAD brand of zopiclone; Temmler brand of zopiclone; Teva brand of zopiclone; Zopiclon AL; Zopiclon AZU; Zopiclon AbZ; Zopiclon Stada; Zopiclon TAD; Zopiclon beta; Zopiclon von ct; RP 27 267; RP 27267; Z 4900; Z4900_SIGMA; Amoban (TN); Ct-Arzneimittel brand of zopiclone; Imovane (TN); Novo-zopiclone; Nu-Pharm brand of zopiclone; Nu-Zopiclone; RP-27267; Ran-zopiclone; Ratio-Zopiclone; Rhone-Poulenc Rorer brand of zopiclone; Zimovane (TN); Zopi-Puren; Zopiclon-TEVA; Zopiclon-neuraxpharm; Zopiclon-ratiopharm; Zopiclona [INN-Spanish]; Zopiclone (TN); Zopiclonum [INN-Latin]; Zopinox (TN); Zopiclone (JAN/INN); Zopiclone [BAN:INN:JAN]; Zopiclone, Imovane, Zimovane, Lunesta; [6-(5-chloropyridin-2-yl)-5-oxo-7H-pyrrolo[3,4-b]pyrazin-7-yl] 4-methylpiperazine-1-carboxylate; 1-Piperazinecarboxylic acid, 4-methyl-, 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo(3,4-b)pyrazin-5-yl ester; 1-Piperazinecarboxylic acid, 4-methyl-, 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl ester; 4-Methyl-1-piperazinecarboxylic acid 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl ester; 4-Methyl-1-piperazinecarboxylic acid ester with 6-(5-chloro-2-pyridyl)-6,7-dihydro-7-hydroxy-5H-pyrrolo(3,4-b)pyrazin-5-one; 6-(5-Chloro-2-pyridinyl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-yl 4-methyl-1-piperazinecarboxylate; 6-(5-chloro-2-pyridyl)-6,7-dihydro-7-oxo-5H-pyrrolo(3,4-b)pyrazin-5-yl 4-methyl-1-piperazinecarboxylate; 6-(5-chloropyrid-2-yl)-5-(4-methylpiperazin-1-yl)carbonyloxy-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazine; 6-(5-chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-yl 4-methylpiperazine-1-carboxylate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
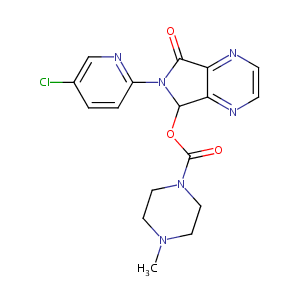 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 388.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Insomnia | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 7A00-7A0Z | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
