Details of the Drug
General Information of Drug (ID: DMQ314B)
| Drug Name |
Hexobarbital
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Barbidorm; Citodon; Citopan; Cyclonal; Cyclopan; Dorico; Enhexymal; Enhexymalum; Esobarbitale; Evipal; Evipan; Hexabarbital;Hexobarbitalum; Hexobarbitone; Hexobarbitonum; Methexenyl; Methylhexabarbital; Methylhexabital; Narcosan; Noctivane; Sombucaps; Sombulex; Somnalert; Esobarbitale [DCIT]; Esobarbitale [Italian]; Hexanastab oral; Sodium hexobarbital; Citopan (TN); Evipan (TN); Hexenal (barbiturate); Hexobarbital (VAN); Hexobarbital [INN:JAN]; Hexobarbitalum [INN-Latin]; Hexobarbital (JAN/INN); N-Methyl-5-cyclohexenyl-5-methylbarbituric acid; (+-)-Hexobarbital; 1,5-Dimethyl-5-(1-cyclohexenyl)barbituric acid; 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-(1-cyclohexen-1-yl)-1,5-dimethyl; 5-(1-CYCLOHEXEN-1-YL)-1,5-DIMETHYLBARBITURIC ACID; 5-(1-Cyclohexen-1-yl)-1,5-dimethyl-2,4,6(1H,3H,5H)-pyrimidinetrione; 5-(1-Cyclohexenyl)-1,5-dimethylbarbituric acid; 5-(1-Cyclohexenyl-1)-1-methyl-5-methylbarbituric acid; 5-(cyclohex-1-en-1-yl)-1,5-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione; 5-(cyclohexen-1-yl)-1,5-dimethyl-1,3-diazinane-2,4,6-trione; 5-(delta-1,2-Cyclohexenyl)-5-methyl-N-methyl-barbitursaeure [German]; 5-(delta-1,2-cyclohexenyl)-5-methyl-N-methyl-barbitursaeure; 5-(delta.-1,2-cyclohexenyl)-5-methyl-N-methyl-barbitursaeure; 5-cyclohex-1-en-1-yl-1,5-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione; 5-cyclohex-1-en-1-yl-2-hydroxy-1,5-dimethylpyrimidine-4,6(1H,5H)-dione
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
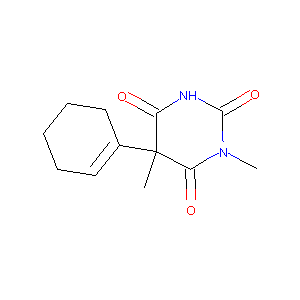 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 236.27 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
References
