Details of the Drug
General Information of Drug (ID: DMSWJ5X)
| Drug Name |
Sildenafil citrate
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Sildenafil (citrate); Sildenafil citrate; Sildenafil citrate [USAN]; Sildenafil citrate salt; UK 92480; UK-92480-10; UNII-BW9B0ZE037; Sildenafilcitrate; Sildenafil; 1-((3-(4,7-Dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo(4,3-d)pyrimidin-5-yl)-4-ethoxyphenyl)sulfonyl)-4-methylpiperazine; 139755-83-2; 3M7OB98Y7H; 5-(2-Ethoxy-5-((4-methylpiperazin-1-yl)sulfonyl)phenyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-7(6H)-one; BNRNXUUZRGQAQC-UHFFFAOYSA-N; C22H30N6O4S; CHEBI:9139; CHEMBL192; HSDB 7305; Sildenafil [INN:BAN]; UK-92,480-10; UK-92480; UNII-3M7OB98Y7H; 1-((3-(6,7-Dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo(4,3-d)pyrimidin-5-yl)-4-ethoxyphenyl)sulfonyl)-4-methylpiperazine citrate (1:1); 171599-83-0; BW9B0ZE037; CHEBI:58987; Caverta; DSSTox_CID_26076; DSSTox_GSID_46076; DSSTox_RID_81321
|
|||||
| Structure |
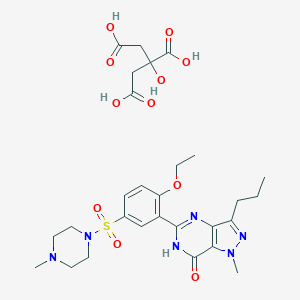 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 3 |
Molecular Weight | 666.7 | ||||
| Logarithm of the Partition Coefficient | Not Available | |||||
| Rotatable Bond Count | 12 | |||||
| Hydrogen Bond Donor Count | 5 | |||||
| Hydrogen Bond Acceptor Count | 15 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
