Details of the Drug
General Information of Drug (ID: DMV2JNY)
| Drug Name |
Cephapirin
|
|||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CEPR; Cefa; Cefadyl; Cefapirin; Cefapirina; Cefapirine; Cefapirinum; Cefaprin; Cephapirine; Metricure; CEPHAPIRIN SODIUM; Cefaprinsodium; Cephapirin Monosodium Salt; ANTIBIOTIC BL-P1322; BL-P 1322; Cefa-ak; Cefadyl (TN); Cefapirin (BAN); Cefapirin [INN:BAN]; Cefapirina [INN-Spanish]; Cefapirine [INN-French]; Cefapirinum [INN-Latin]; Metricure (TN); (6R,7R)-3-(Acetoxymethyl)-8-oxo-7-(2-(4-pyridylthio)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-en-2-carbonsaeure; (6R,7R)-3-(acetyloxymethyl)-8-oxo-7-[(2-pyridin-4-ylsulfanylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-[(acetyloxy)methyl]-8-oxo-7-{[(pyridin-4-ylthio)acetyl]amino}-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-acetoxymethyl-7-[(pyridin-4-ylsulfanyl)acetamido]-3,4-didehydrocepham-4-carboxylic acid; 3-(Hydroxymethyl)-8-oxo-7-(2-(4-pyridylthio)acetamidol-5-thia-1-azabicyclo(4.2.0)oct-2-en-2carbonsaeure acetat; 5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 3-(hydroxymethyl)-8-oxo-7-(2-(4-pyridylthio)acetamido)-, acetate (ester); 7-(2-(4-Pyridylthio)acetamido)cephalosporanic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
|||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
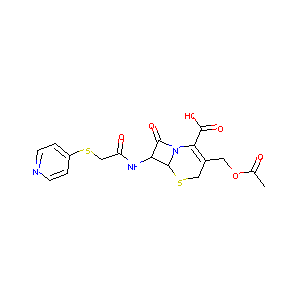 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 423.5 | ||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.1 | |||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | |||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Cephapirin (Comorbidity)
|
|||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Cephapirin FDA Label | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 062720. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | The formation of functional penicillin-binding proteins. J Biol Chem. 1975 Aug 25;250(16):6578-85. | ||||
| 7 | Engle JE, Drago J, Carlin B, Schoolwerth AC "Letter: Reversible acute renal failure after cephalothin." Ann Intern Med 83 (1975): 232-3. [PMID: 1147461] | ||||
