Details of the Drug
General Information of Drug (ID: DMVDTR2)
| Drug Name |
Thymoquinone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
490-91-5; Thymoquinon; p-Cymene-2,5-dione; 2-Isopropyl-5-methyl-1,4-benzoquinone; 2,5-CYCLOHEXADIENE-1,4-DIONE, 2-METHYL-5-(1-METHYLETHYL)-; 2-Isopropyl-5-methyl-p-benzoquinone; 2-Isopropyl-5-methylbenzoquinone; Polythymoquinone; 5-Isopropyl-2-methyl-1,4-benzoquinone; 2-Isopropyl-5-methylbenzo-1,4-quinone; p-Mentha-3,6-diene-2,5-dione; NSC 2228; 2-Isopropyl-5-methylcyclohexa-2,5-diene-1,4-dione; 2-Methyl-5-isopropyl-p-benzoquinone; 2-methyl-5-propan-2-ylcyclohexa-2,5-diene-1,4-dione; NSC2228; 2-methyl-5-(propan-2-yl)cyclohexa-2,5-diene-1,4-dione; UNII-O60IE26NUF; 2-Methyl-5-isopropyl-1,4-benzoquinone; O60IE26NUF; 2,5-Cyclohexadiene-1,4-dione, 5-isopropyl-2-methyl-; NSC-2228; 5-Isopropyl-2-methyl-p-benzoquinone; MFCD00001602; 2-Methyl-5-(1-methylethyl)-2,5-cyclohexadiene-1,4-dione; p-Mentha-3,6-diene-2,5-dione (8CI); 5-Isopropyl-2-methyl-2,5-Cyclohexadiene-1,4-dione; CCRIS 7152; EINECS 207-721-1; 2-methyl-5-(methylethyl)cyclohexa-2,5-diene-1,4-dione; BRN 1939047; thymolquinone; Thymoil; AI3-17758; 4hco; p-Mentha-3,5-dione; Spectrum_001237; SpecPlus_000457; Thymoquinone, >=98%; Spectrum2_000700; Spectrum3_001345; Spectrum4_001895; Spectrum5_000550; BSPBio_003129; KBioGR_002455; KBioSS_001717; DivK1c_006553; SCHEMBL542535; SPBio_000859; CHEMBL1672002; DTXSID9060079; KBio1_001497; KBio2_001717; KBio2_004285; KBio2_006853; KBio3_002349; Thymoquinone, analytical standard; CHEBI:113532; 2-Methyl-5-iso-propylbenzoquinone; BDBM166686; ZINC164367; BCP16946; HY-D0803; WLN: L6V DVJ B1 EY1&1; 2,4-dione, 5-isopropyl-2-methyl-; ANW-41600; CCG-40027; s4761; SBB008296; AKOS003368628; MCULE-9899033250; NCGC00178250-01; NCGC00178250-05; 73940-92-8; AK101679; AS-11327; 2-Isopropyl-5-methylbenzo-1,4-quinone #; 2,4-dione, 2-methyl-5-(1-methylethyl)-; CS-0012226; FT-0612708; ST45023960; K-9199; SR-05000002192; Q7799650; SR-05000002192-2; W-202869; BRD-K97566842-001-03-5; Thymoquinone; 2-isopropyl-5-methylbenzo-1,4-quinone; 2-methyl-5-(propan-2-yl)cyclohexa-2,5-diene-1,4-dione (F8); 2-Methyl-5-(1-methylethyl)-2,5-cyclohexadiene-1,4-dione, 9CI
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
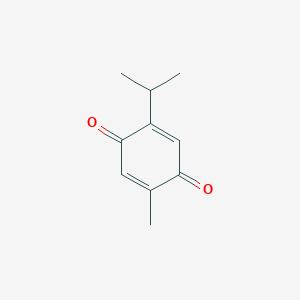 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 164.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Polycystic ovarian syndrome | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A80.1 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
