Details of the Drug
General Information of Drug (ID: DMX9K8F)
| Drug Name |
Carbachol
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Carbach; Carbacholin; Carbacholine; Carbacholinum; Carbacholum; Carbacol; Carbacolina; Carbacolo; Carbamiotin; Carbamoylcholine; Carbastat; Carbochol; Carbocholin; Carbocholine; Carbyl; Carcholin; Coletyl; Doryl; Jestryl; Karbachol; Lentin; Lentine; Miostat; Moryl; Rilentol; Vasoperif; CARBACHOL CHLORIDE; Carbachol hydrochloride; Carbacholine chloride; Carbacholini chloridum; Carbacholinium chloratum; Carbacholum chloratum; Carbacolo [DCIT]; Carbaminocholine chloride; Carbaminoylcholine chloride; Carbamoylcholine chloride; Carbamylcholine chloride; Choline carbamate chloride; Choline chloride carbamate; Choline chlorine carbamate; Isopto Carbachol; Karbachol [Czech]; Karbamoylcholin chlorid; Karbamoylcholin chlorid [Czech]; Lentine [French]; Mistura C; C 4382; TL 457; C-1770; Carbacholum [INN-Latin]; Carbacol [INN-Spanish]; Carbamic acid, ester with choline chloride; Carbamoylcholine-hydrochloride; Carbastat (TN); Carboptic (TN); Choline chloride, carbamate; Doryl (VAN); Doryl (pharmaceutical); Gamma-Carbamoyl choline chloride; Isopto Carbachol (TN); Miostat (TN); Carbachol [INN:BAN:JAN]; Choline, chloride carbamate(ester); Choline, chloride, carbamate; P. V. Carbachol; Carbachol (JAN/USP/INN); Choline, chloride, carbamate, hydrochloride; Ethanaminium, 2-(aminocarbonyl)oxy-N,N,N-trimethyl-, chloride; Ethanaminium, 2-[(aminocarbonyl)oxy]-N,N,N-trimethyl-, chloride; Ethanaminium, 2-((aminocarbonyl)oxy)-N,N,N-trimethyl-, chloride; Ethanaminium, 2-((aminocarbonyl)oxy)-N,N,N-trimethyl-, chloride (1:1); (2-Carbamoyloxy-ethyl)-trimethyl-ammonium; (2-Carbamoyloxy-ethyl)-trimethyl-ammonium(Carbachol); (2-Carbamoyloxyethyl)trimethylammonium chloride; (2-Hydroxyethyl)trimethyl ammonium chloride carbamate; (2-Hydroxyethyl)trimethylammonium chloride carbamate; (carbachol)(2-Carbamoyloxy-ethyl)-trimethyl-ammonium; 2-((Aminocarbonyl)oxy)-N,N,N-trimethylethanaminium chloride; 2-((Aminocarbonyl)oxy)-N,N,N-trimethylethanaminum chloride; 2-(carbamoyloxy)-N,N,N-trimethylethanaminium chloride; 2-[(aminocarbonyl)oxy]-N,N,N-trimethylethanaminium chloride; 2-carbamoyloxyethyl(trimethyl)azanium chloride
|
|||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Cardiotonic Agents
|
|||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||
| Structure |
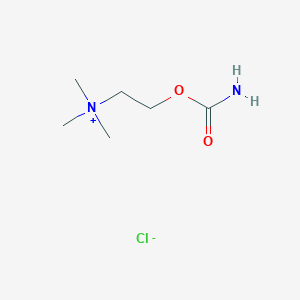 |
|||||||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | |||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 182.65 | ||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | |||||||||||||||||||||||||||||||||||
| Rotatable Bond Count | 4 | |||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | |||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 3 | |||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 298). | ||||
|---|---|---|---|---|---|
| 2 | Rodrigues WC, Castro C, Catbagan P, Moore C, Wang G: Immunoassay screening of diphenhydramine (Benadryl(R)) in urine and blood using a newly developed assay. J Anal Toxicol. 2012 Mar;36(2):123-9. doi: 10.1093/jat/bkr015. | ||||
| 3 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 4 | Synergy between pairs of competitive antagonists at adult human muscle acetylcholine receptors. Anesth Analg. 2008 Aug;107(2):525-33. | ||||
| 5 | Molecular basis of inhibition of substrate hydrolysis by a ligand bound to the peripheral site of acetylcholinesterase. Chem Biol Interact. 2010 Sep 6;187(1-3):135-41. doi: 10.1016/j.cbi.2010.05.009. Epub 2010 May 21. | ||||
| 6 | Histamine H1 receptor induces cytosolic calcium increase and aquaporin translocation in human salivary gland cells. J Pharmacol Exp Ther. 2009 Aug;330(2):403-12. | ||||
| 7 | Regulation of antiprotease and antimicrobial protein secretion by airway submucosal gland serous cells. J Biol Chem. 2004 Sep 10;279(37):38854-60. doi: 10.1074/jbc.M407077200. Epub 2004 Jul 2. | ||||
| 8 | Enhanced proliferation of SNU-407 human colon cancer cells by muscarinic acetylcholine receptors. BMB Rep. 2008 Nov 30;41(11):803-7. doi: 10.5483/bmbrep.2008.41.11.803. | ||||
| 9 | Regional differences in functional receptor distribution and calcium mobilization in the intact human lens. Invest Ophthalmol Vis Sci. 2001 Sep;42(10):2355-63. | ||||
| 10 | Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol Pharmacol. 1997 Jul;52(1):172-9. | ||||
| 11 | Zinc oxide nanoparticle disruption of store-operated calcium entry in a muscarinic receptor signaling pathway. Toxicol In Vitro. 2010 Oct;24(7):1953-61. doi: 10.1016/j.tiv.2010.08.005. Epub 2010 Aug 12. | ||||
