Details of the Drug
General Information of Drug (ID: DMXQS7K)
| Drug Name |
Cenestin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Sodium equilin sulfate; Equilin sodium sulfate; Climopax; Estratab; 16680-47-0; Sodium equilin 3-monosulfate; Emopremarin; Novoconestron; Climestrone; Promarit; Primarin; Premarose; Premaril; Menotrol; Ganeake; Estropan; Estrifol; Premarina; Palopause; Menogen; Mannest; Hyphorin; Formatrix; Estromed; Equigyne; Prempak; Oestrilin; Menotab; Kestrin; Glyestrin; Estroate; Equilin 3-Sulfate Sodium Salt; Conjugen; Conestron; Ayerogen; Theogen; Sukingpo
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
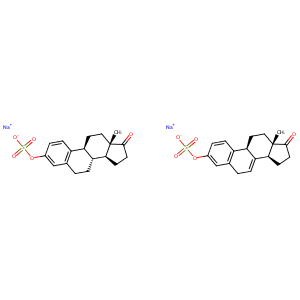 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 370.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||||||
| Rotatable Bond Count | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 5 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Menopause symptom | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | GA30.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Estrogen replacement therapy and cardioprotection: mechanisms and controversies. Braz J Med Biol Res. 2002 Mar;35(3):271-6. | ||||
| 3 | Relationship between the angiotensin-converting enzyme genotype and the forearm vasodilator response to estrogen replacement therapy in postmenopausal women. J Am Coll Cardiol. 2001 May;37(6):1529-35. doi: 10.1016/s0735-1097(01)01191-3. | ||||
| 4 | Circulating chemoattractants RANTES, negatively related to endogenous androgens, and MCP-1 are differentially suppressed by hormone therapy and raloxifene. Atherosclerosis. 2007 Jul;193(1):142-50. doi: 10.1016/j.atherosclerosis.2006.05.045. Epub 2006 Jul 13. | ||||
| 5 | Effects of estrogen, raloxifene, and hormone replacement therapy on serum C-reactive protein and homocysteine levels. Maturitas. 2006 Feb 20;53(3):252-9. doi: 10.1016/j.maturitas.2005.05.006. Epub 2005 Jun 28. | ||||
| 6 | Short-term effects of estrogen, tamoxifen and raloxifene on hemostasis: a randomized-controlled study and review of the literature. Thromb Res. 2005;116(1):1-13. doi: 10.1016/j.thromres.2004.09.014. | ||||
| 7 | Estrogens exert route- and dose-dependent effects on insulin-like growth factor (IGF)-binding protein-3 and the acid-labile subunit of the IGF ternary complex. J Clin Endocrinol Metab. 2000 May;85(5):1918-22. doi: 10.1210/jcem.85.5.6527. | ||||
