| 1 |
ClinicalTrials.gov (NCT01889602) Characterizing and Predicting Drug Effects on Cognition
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6849).
|
| 3 |
Topiramate FDA Label
|
| 4 |
The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954.
|
| 5 |
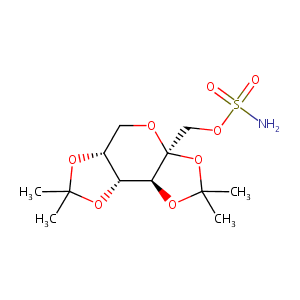
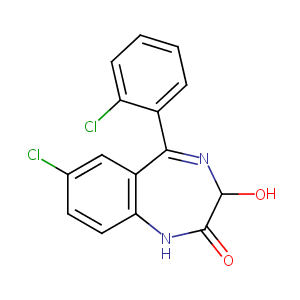
Lorazepam FDA Label
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5884).
|
| 7 |
The association between the C802T polymorphism of the UDP-glucuronosyltransferase 2B7 gene and effective topiramate dosage. Zh Nevrol Psikhiatr Im S S Korsakova. 2007;107(5):63-4.
|
| 8 |
Development of medications for alcohol use disorders: recent advances and ongoing challenges. Expert Opin Emerg Drugs. 2005 May;10(2):323-43.
|
| 9 |
The antiepileptic drug topiramate is a substrate for human P-glycoprotein but not multidrug resistance proteins. Pharm Res. 2009 Nov;26(11):2464-70.
|
| 10 |
Dose-dependent induction of cytochrome P450 (CYP) 3A4 and activation of pregnane X receptor by topiramate. Epilepsia. 2003 Dec;44(12):1521-8.
|
| 11 |
A preliminary pharmacogenetic investigation of adverse events from topiramate in heavy drinkers. Exp Clin Psychopharmacol. 2009 Apr;17(2):122-9. doi: 10.1037/a0015700.
|
| 12 |
Effects of anticonvulsants on human p450c17 (17alpha-hydroxylase/17,20 lyase) and 3beta-hydroxysteroid dehydrogenase type 2. Epilepsia. 2005 Mar;46(3):444-8.
|
| 13 |
Design, synthesis, and biological evaluation of novel carbohydrate-based sulfamates as carbonic anhydrase inhibitors. J Med Chem. 2011 Mar 10;54(5):1481-9.
|
| 14 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 15 |
Effects of the antiepileptic drugs lamotrigine, topiramate and gabapentin on hERG potassium currents. Epilepsy Res. 2005 Jan;63(1):17-25. doi: 10.1016/j.eplepsyres.2004.10.002. Epub 2004 Dec 8.
|
| 16 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 17 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 18 |
Pharmacokinetic and pharmacodynamic interaction of lorazepam and valproic acid in relation to UGT2B7 genetic polymorphism in healthy subjects. Clin Pharmacol Ther. 2008 Apr;83(4):595-600.
|
| 19 |
Effect of the UGT2B15 genotype on the pharmacokinetics, pharmacodynamics, and drug interactions of intravenous lorazepam in healthy volunteers. Clin Pharmacol Ther. 2005 Jun;77(6):486-94.
|
|
|
|
|
|
|