| 1 |
ClinicalTrials.gov (NCT05249114) Study of Cabozantinib With Lu-177 in Patients With Somatostatin Receptor 2 Positive Neuroendocrine Tumors
|
| 2 |
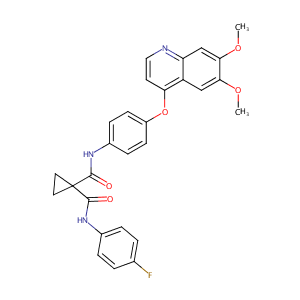
Cabozantinib FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5887).
|
| 4 |
2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89.
|
| 5 |
Clinical pipeline report, company report or official report of Exelixis (2011).
|
| 6 |
Practical management of adverse events associated with cabozantinib treatment in patients with renal-cell carcinoma. Onco Targets Ther. 2017 Oct 19;10:5053-5064.
|
| 7 |
FDA Label of Cabozantinib. The 2020 official website of the U.S. Food and Drug Administration.
|
| 8 |
ROS-dependent DNA damage contributes to crizotinib-induced hepatotoxicity via the apoptotic pathway. Toxicol Appl Pharmacol. 2019 Nov 15;383:114768. doi: 10.1016/j.taap.2019.114768. Epub 2019 Oct 19.
|
| 9 |
Cytotoxicity of 34 FDA approved small-molecule kinase inhibitors in primary rat and human hepatocytes. Toxicol Lett. 2018 Jul;291:138-148. doi: 10.1016/j.toxlet.2018.04.010. Epub 2018 Apr 12.
|
|
|
|
|
|
|